Full Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
PAKISTAN. Sajid Ali1-2, Peter Biermanns1, Rashid Haider3 and Klaus Reicherter1 5 1Neotectonics and Natural Hazards, RWTH Aachen University, Lochnerstr
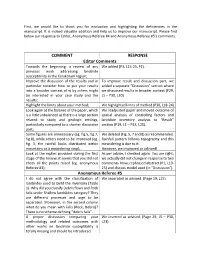
First, we would like to thank you for evaluation and highlighting the deficiencies in the manuscript. It is indeed valuable addition and help us to improve our manuscript. Please find below our response to Editor, Anonymous Referee #4 and Anonymous Referee #5’s comments. COMMENT RESPONSE Editor Comments Towards the beginning, a review of any We added (P3, L15-25, P2). previous work addressing landslide susceptibility in the Karakoram region; Improve the discussion of the results and in To improve result and discussion part, we particular consider how to put your results added a separate “Discussion” section where into a broader context of w by others might we discussed results in broader context (P29, be interested in your case study and the L5 – P30, L30) results; Highlight the limits about your method; We highlighted limits of method (P30, L18-24) Look again at the balance of the paper, which We readjusted paper and moved outcome of is a little unbalanced as there is a large section spatial analysis of controlling factors and related to study and geologic settings, landslide inventory analysis to “Result” particularly compared to a shorter discussion section (P19, L1 – P23, L20). part; Some figures are unnecessary (eg. fig.5, fig.7, We deleted (Fig. 5, 7 and 8) as recommended. fig.8), while others need to be improved (eg. Rainfall pattern follows topography and this fig. 3, the rainfall looks distributed within meandering is due to it. mountains as a meandering strip); However, we improved as advised! Look at the replies provided during the first As per advice, I checked again. -

Hydel Power Potential of Pakistan 15
Foreword God has blessed Pakistan with a tremendous hydel potential of more than 40,000 MW. However, only 15% of the hydroelectric potential has been harnessed so far. The remaining untapped potential, if properly exploited, can effectively meet Pakistan’s ever-increasing demand for electricity in a cost-effective way. To exploit Pakistan’s hydel resource productively, huge investments are necessary, which our economy cannot afford except at the expense of social sector spending. Considering the limitations and financial constraints of the public sector, the Government of Pakistan announced its “Policy for Power Generation Projects 2002” package for attracting overseas investment, and to facilitate tapping the domestic capital market to raise local financing for power projects. The main characteristics of this package are internationally competitive terms, an attractive framework for domestic investors, simplification of procedures, and steps to create and encourage a domestic corporate debt securities market. In order to facilitate prospective investors, the Private Power & Infrastructure Board has prepared a report titled “Pakistan Hydel Power Potential”, which provides comprehensive information on hydel projects in Pakistan. The report covers projects merely identified, projects with feasibility studies completed or in progress, projects under implementation by the public sector or the private sector, and projects in operation. Today, Pakistan offers a secure, politically stable investment environment which is moving towards deregulation -

Draft Report on KAP Studies (GB and KPK) August 3, 2020
Draft Report – Knowledge, Attitude and Practices KAP Studies as well as Documenting Local / Indigenous Knowledge for 15 Districts of KP and GB I | P a g e TABLE OF CONTENTS Index of Tables ..................................................................................................................................... VI Index of Figures .................................................................................................................................. VII Acronyms .............................................................................................................................................. IX Executive Summary ............................................................................................................................... X 1. Background ..................................................................................................................................... 1 1.1. Objectives of KAP .................................................................................................................. 1 2. Implementation Strategy ................................................................................................................. 2 2.1. Inception Meeting ................................................................................................................... 2 2.2. Review of Literature ............................................................................................................... 2 2.3. Development of Research Tools ............................................................................................ -

PLF – 1St June 2021 NO MASK NO ENTRY! Fatima Jinnah Govt
PAKISTAN LEARNING FESTIVAL GILGIT BALTISTAN 2021 | June 1-3, 2021 “Celebrating & Preserving the Multicultural Heritage & Eco Diversity of Gilgit Baltistan” Day 1 – PLF – 1st June 2021 NO MASK NO ENTRY! Fatima Jinnah Govt. Degree College for Women in Gilgit Welcoming “Back to School” as a rich Social Emotional Learning Experience Registration 8:45am -9:30 a.m. COVID- SOPs Kiosks. Let’s Remain Healthy by Being Safe! (Masks/Hygiene/Sanitizers) Location Time 9:30am-10:30am 10:45am-11:45am 12:00pm - 1:00pm 1:15pm - 2:15pm 2:30pm-3:30pm INAUGURAL Tilawat & National Anthem (in PSL) A Conversation on Special Storytelling with Photographs –“where did Short Films for Social Change and Storytelling Musical Dadi Jawari (Main Auditorium) CLF Taraana – “Hamain Kitab Chahiye”: Lyrics: Zehra Nigah, Olympics and Young Athletes all the butterflies go?” by Arif Amin Films in the Classrooms Performance by program of Charpursan Valley By Samar Minallah Kashif Din At Welcome to The Magic of PLF Baela Raza Jamil - Back to School – Theater and Panel discussions Panel discussion with Olympic Comments by: Umbreen Arif & Afia Salam Comments: Dr. Fouzia Khan – Chief Adviser Dadi Jawari Celebration Champions, “Najeeba Zareen & Curriculum SELD Main Tributes to Legends: Farzana Rehmat Ali” Auditorium Our Legends lost: 2020-21(Literature etc.) Ali Sadpara & Award Moderated by: Ahmed Baig Incredible Sisters “Najeeba Zareen & Farzana Rehmat Ali” of Storytelling: Stories in Local Languages by Nazir A. Book Launch – from the Mini Library stories from Deosai (Room 1) Charpursan Valley GB StoryTelling A Children’s History of Gilgit- Bulbul (Bulbulik Heritage Centre) ( 40 mins) 10 countries in Urdu; Pakistan Literacy Project Celebrating languages and Literacy Karishma Aziz- Winner CLF Young Author Award 2021 Baltistan by Tooba Malik Storytelling: Dr. -

Trade, Development, and Control in Western China Borderland Infrastructures Asian Borderlands
ASIAN BORDERLANDS Alessandro Rippa Borderland Infrastructures Trade, Development, and Control in Western China Borderland Infrastructures Asian Borderlands Asian Borderlands presents the latest research on borderlands in Asia as well as on the borderlands of Asia – the regions linking Asia with Africa, Europe and Oceania. Its approach is broad: it covers the entire range of the social sciences and humanities. The series explores the social, cultural, geographic, economic and historical dimensions of border-making by states, local communities and flows of goods, people and ideas. It considers territorial borderlands at various scales (national as well as supra- and sub-national) and in various forms (land borders, maritime borders), but also presents research on social borderlands resulting from border-making that may not be territorially fixed, for example linguistic or diasporic communities. Series Editors Tina Harris, University of Amsterdam Willem van Schendel, University of Amsterdam Editorial Board Members Franck Billé, University of California, Berkeley Duncan McDuie-Ra, University of New South Wales Eric Tagliacozzo, Cornell University Yuk Wah Chan, City University Hong Kong Borderland Infrastructures Trade, Development, and Control in Western China Alessandro Rippa Amsterdam University Press The research, writing, and editing of this volume was supported by generous grants and fellowships from the University of Aberdeen (Research Project Award “Religion and Politics in the Contemporary World”), the European Research Council (ERC -

Pakistan Autumn Sightseeing Tour
Pakistan Autumn Sightseeing Tour Tour Highlights • Karakoram Highway –Old Silk Route • Eye-Catching Beauty of Nanga Parbat-8125m • Famous Indus River & visit Junction of Karakoram, Himalaya, Hindukush • Hunza Valley and views of snowcapped Rakaposhi 7788m • Explore Kalash valleys of Chitral • Famous Passes- Khunjerab, Shandoor and Lowari Explore All Provinces by driving through Besham,Islamabad, Lahore, Multan, Sukkur 20 NIGHTS HOTEL, 21 DAYS US$1100/PAX FOR A GROUP OF 12 SIGHTSEEING US$ 1200/PAX FOR A GROUP OF 10 SOFT HIKING US$1350/PAX FOR A GROUP OF 8-9 MAX. ALTITUDE – 4733M / 15,528FT US$1450/PAX FOR A GROUP OF 5-7 Key Destinations: Lahore - Islamabad- Karakoram Highway - Gilgit- Hunza Khunjerab Pass- Ghizer Valley- Shandoor Pass-Chitral-Kalash Valley-Lowari Pass www.snowland.com.pk PK: +92(0)3335105022 [email protected] 2 | Page Pakistan Autumn Sightseeing Tour TOUR BACKGROUND This exciting tour will take you to the dramatic and enchanting valleys of the Northern Areas (Gilgit-Baltistan & Chitral) of Pakistan. Lush green fertile valleys with high quality delicious fruit are fed by springs and glacial streams flowing from the lofty snow capped mountains. Karakoram Highway and River Indus is a constant Feature of the region. The best time of year to visit Hunza and Chitral is April to October. From spring to autumn the valleys are more attractive, all three fabulous seasons have their own unique charm, which cannot be described in words but can only be felt. TOUR OVERVIEW DEPARTURE DATES: April- November 2018 Date Day Activity -

Hydro Power Resources of Pakistan (I) Private Power and Infrastructure Board
Private Power and Infrastructure Board Foreword Pakistan is endowed with plenty of natural resources, including water resources. Besides water supply and irrigation, water resources are also utilized to produce electricity. National water resources have rich potential for hydropower generation, estimated as 60000 MW which could be economically harnessed. Out of this vast hydropower potential only 11% has been developed so far. Hydropower is the best available option in the recent scenario of meeting challenges of projected future energy demands of our country as it is sustainable, reliable, renewable, clean, low cost and indigenous thus can be the principal source of energy. It is therefore imperative to put all-out efforts towards development of the untapped hydropower potential without further delay. Accordingly, there is a transition in policy priority i.e. shifting from development of gas/oil based thermal power plants with merits of comparatively shorter construction time and lower capital investment to hydropower generation. The Private Power and Infrastructure Board (PPIB) has already been successful in attracting significant investment in developing hydropower projects, which are currently at different stages of implementation. The world-over, hydropower projects are characterized with a variety of technical and economic constraints and bottlenecks, Pakistan being no exception. These include hydrological risks, resettlement and environmental issues, regulatory matters, market dynamics and financing problems. In the past no attention was ever paid to address these impediments in development of hydropower projects. The government has now taken a number of initiatives to remove all the factors hampering Independent Power Producers (IPPs) in developing hydropower projects. Focus is on creating synergy among various stakeholders including regulatory bodies. -

Analysis of Landslide Movements Using Interferometric Synthetic Aperture Radar: a Case Study in Hunza-Nagar Valley, Pakistan
remote sensing Article Analysis of Landslide Movements Using Interferometric Synthetic Aperture Radar: A Case Study in Hunza-Nagar Valley, Pakistan Mohib Ur Rehman 1,2, Yi Zhang 3,*, Xingmin Meng 3, Xiaojun Su 1 , Filippo Catani 4 , Gohar Rehman 5, Dongxia Yue 1, Zainab Khalid 1, Sajjad Ahmad 5 and Ijaz Ahmad 6 1 College of Earth and Environmental Sciences, Lanzhou University, Lanzhou 730000, China; [email protected] (M.U.R.); [email protected] (X.S.); [email protected] (D.Y.); [email protected] (Z.K.) 2 Department of Earth Sciences, COMSATS University Islamabad-Abbottabad Campus, Abbottabad 22060, Pakistan 3 School of Earth Sciences, Lanzhou University, Lanzhou 730000, China; [email protected] 4 Earth Science Department, University of Florence, 50121 Firenze, Italy; filippo.catani@unifi.it 5 Department of Geology, University of Peshawar, Peshawar 25210, Pakistan; [email protected] (G.R.); [email protected] (S.A.) 6 Pakistan Atomic Energy Commission, Islamabad 1114, Pakistan; [email protected] * Correspondence: [email protected] Received: 14 May 2020; Accepted: 22 June 2020; Published: 25 June 2020 Abstract: From a geological standpoint, northern Pakistan is one of the most active and unstable areas in the world. As a consequence, many massive landslides have occurred in the area in historical times that have destroyed infrastructure, blocked the Hunza River, and damaged the Karakoram Highway repeatedly. However, despite the high frequency of large magnitude landslide events, and the consequent damages, the entire area is largely understudied, mainly due to the difficult logistics and the large distances involved. This work is aimed at applying the potential use of Interferometric Synthetic Aperture Radar (InSAR) for landslide identification and investigation for the Hunza-Nagar Region. -

Understanding the Transboundary Karakoram-Pamir Landscape
Feasibility and Baseline Studies #1 Understanding the Transboundary Karakoram-Pamir Landscape 1 Feasibility and Baseline Studies #1 Understanding the Transboundary Karakoram-Pamir Landscape Wu Ning, Muhammad Ismail, Srijana Joshi, Faisal M Qamar, Karma Phuntsho, Yang Weikang, Babar Khan, Yi Shaoliang, Rajan Kotru, and Eklabya Sharma International Centre for Integrated Mountain Development, Kathmandu, Nepal, November 2014 Published by International Centre for Integrated Mountain Development GPO Box 3226, Kathmandu, Nepal Copyright © 2014 International Centre for Integrated Mountain Development (ICIMOD) All rights reserved. Published 2014 ISBN 978 92 9115 326 8 (printed) 978 92 9115 327 5 (electronic) LCCN 2014-347287 Production team A Beatrice Murray (Consultant editor) Amy Sellmyer (Editor) Sushil Dhungana (Consultant Graphic designer) Asha Kaji Thaku (Editorial assistant) Printed and bound in Nepal by Quality Printers Pvt. Ltd, Kathmandu, Nepal Note This publication may be reproduced in whole or in part and in any form for educational or non-profit purposes without special permission from the copyright holder, provided acknowledgement of the source is made. ICIMOD would appreciate receiving a copy of any publication that uses this publication as a source. No use of this publication may be made for resale or for any other commercial purpose whatsoever without prior permission in writing from ICIMOD. The views and interpretations in this publication are those of the author(s). They are not attributable to ICIMOD and do not imply the expression of any opinion concerning the legal status of any country, territory, city or area of its authorities, or concerning the delimitation of its frontiers or boundaries, or the endorsement of any product. -

Hunza/Gilgit the Travel Explorers Break Fast at Hotel and Travel to Gilgit
EXPLORE PAKISTAN 5 Days Hunza Tour Tour Highlighs Rakaposhi View Point Altit & Baltit Fort Attabad Lake Passu Cones Hussaini Bridge Khunjrab paas Nagar Valley Hopper Glaciers Duiker Valley Chinies Graveyard NLI Market The Travel Explorers Day - 01 Islamabad/ Gilgit / Hunza The Travel Explorers Departure from Islamabad to Gilgit by flight. Meet & greet @ the airport transfer to Hunza. It’s a 03-Hours Scenic Drive on Economic Corridor; a short stop at Rakaposhi view point Restaurant with breathtaking view of Rakaposhi Mountain. It's a beautiful place to spend 30 minutes on the way to Hunza. After arrival in Hunza transfer to Hotel. Afternoon Visit to Altit Fort & Baltit Fort. Altit fort is situated in the village of Altit about three kilometers from Karimbabd Hunza. It has been built on a sheer rock cliff that falls 300 meters (1,000 feet) into the Indus river. The fort is a 100 years older than the Baltit Fort and wears at one time inhabited by the ruling family. Today there is a museum built within the Fort for the tourist. A trip to the Baltit & Altit is must while your tour to Hunza Valley. Baltit Fort; Built in Eleventh-century, located 2800 m to the summits of the Himalayas, was built at the time, enemies and withstands frequent earthquakes. Until 1950, the fort was the residence of the Mirs of Hunza, but was then left to perish. The recent restoration, proposed by the Aga Khan Trust has carefully respecting the original construction techniques. Although rain is rare in the Hunza valley; still is irrigated by an ingenious system of canals fed by ancient glaciers. -

Gender and Environmental Migration in the Karakoram Region
Giovanna Gioli Field Trip Report: Gender and Environmental Migration in the Karakoram Region University of Hamburg Research Group Climate Change and Security Working Paper CLISEC-24 Field Trip Report: Gender and Environmental Migration in the Karakoram Region Giovanna Gioli1 (1) Research Fellow at the Research Group Climate Change and Security (CLISEC), KlimaCampus, Institute of Geography, University of Hamburg, Grindelberg 7, #2012, D‐20144, Hamburg, Germany. Tel: +49 40 42838‐9196 Email: [email protected] Background The “Gender and Environmental Migration” (GEM) project is the result of a joint collaboration between the CliSAP’s Research Group “Climate Change and Security” (CLISEC) of the University of Hamburg, and the Islamabad‐based think‐tank “Sustainable Development Policy Institute” (SDPI), under the MOU recently signed by the SDPI director, Dr. Abid Q. Suleri, and the Dean of the Faculty of Mathematics, Informatics and Natural Sciences, Professor Heinrich Graener. The project aims at collecting gender disaggregated data on local perceptions of climate change and variability, and on adaptation strategies to climate change impacts in the Karakoram region, with a special focus on migration as adaptive strategy and its gendered impacts. After a preliminary exploratory trip to Pakistan (February 24th‐March 11th 2012), aimed at assessing the feasibility of the study, I spent two months in Pakistan (May/June 2012). During the first month I was based at SDPI in Islamabad, developing the research tools and conducting preliminary interviews with key stakeholders. The following people have been interviewed: Name Affiliation Website Mr. Shazad Director of the Gilgit‐Baltistan http://www.hunzaalps.com/epa/ Hasan Shigri Environmental Protection Agency (EPA) Mr. -
The Good Son Letters How a Swiss-Born, Twice-Married Socialite
NES-15 PAKISTAN Nicholas Schmidle is a Phillips Talbot Fellow of the ICWA Institute studying identity and politics in Pakistan. The Good Son LETTERS How a Swiss-born, twice-married socialite Since 1925 the Institute of became leader of the Muslims and formed Current World Affairs (the Crane- the world’s largest, borderless welfare state Rogers Foundation) has provided long-term fellowships to enable By Nicholas Schmidle outstanding young professionals JUNE 2007 to live outside the United States and write about international IN GULKIN, A VILLAGE NESTLED HIGH in the Karakorum Mountains areas and issues. An exempt of northern Pakistan, Ghulam Karim and his team of volunteers had already mixed and poured more than 50 bags of concrete by the middle of the afternoon on a recent operating foundation endowed summer day. The team consisted of several dozen men, ranging from teenagers to by the late Charles R. Crane, grandfathers. The older ones wore toppis, traditional, floppy wool caps. They pulled the Institute is also supported by shoebox-sized stones from one pile and threw them onto another, chitchatting the contributions from like-minded entire time. Excited and jumpy as they pushed their wheelbarrows back and forth, individuals and foundations. the younger ones shuttled loads from the soppy pile of wet mix to the patio area under construction. They laughed, joked, and, on more than one occasion, turned the water hose on each other. They were preparing for a party. TRUSTEES Bryn Barnard Ghulam, a 42-year-old man with civic spirit reminiscent of a Little League Carole Beaulieu baseball coach, stood covered in concrete dust.