Fexofenadine Hydrochloride) Capsules and Tablets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

22-556Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 22-556Orig1s000 MEDICAL REVIEW(S) MEDICAL OFFICER REVIEW Division Of Pulmonary, Allergy, and Rheumatology Products, HFD-570 APPLICATION: NDA 22-556 TRADE NAME: Karbinal ER™ APPLICANT/SPONSOR: Tris Pharma USAN NAME: Carbinoxamine Extended-Release MEDICAL OFFICER: Peter Starke, MD Oral Suspension TEAM LEADER: Theresa Michele, MD CATEGORY: Antihistamine DATE: February 25, 2013 ROUTE: Oral SUBMISSIONS REVIEWED IN THIS DOCUMENT Document Date Submission Date Application/Doc Comments October 4, 2012 October 5, 2012 SD-17 Complete Response submission January 8, 2013 January 9, 2013 SD-20 Response to labeling (formatting) IR RELATED APPLICATIONS Date Application Comments REVIEW SUMMARY: This is clinical review of a Complete Response (CR) to a CR action taken by the Agency on October 7, 2011, for a 505(b)(2) application from Tris Pharma for Carbinoxamine Extended-Release (ER) Oral Suspension, equivalent to 4 mg of carbinoxamine maleate (CM) per 5 mL. The formulation is a sustained release formulation of carbinoxamine maleate suspended in a drug-polistirex resin complex. The proposed Trade Name is Karbinal ER. The application references both the currently available generic immediate-release Carbinoxamine Maleate 4 mg tablets (ANDA 40-442) and oral solution 4 mg/5 mL(ANDA 40-458), marketed under the brand name Palgic and manufactured by Milkart, Inc., and the no-longer-marketed immediate-release innovator products, Clistin 4 mg tablets (NDA 08-915) and 4 mg/5 mL elixir (NDA 08-955), previously marketed by McNeil. McNeil discontinued marketing the Clistin products in the 1990s, and the Orange Book makes the notation that the Clistin products were not discontinued or withdrawn for safety or efficacy reasons. -

FEXOFENADINE HYDROCHLORIDE 180 MG FILM-COATED TABLETS Fexofenadine Hydrochloride
ID1089 MRP _ UK Version: 07 Review Date: 19/02/2020 PACKAGE LEAFLET: INFORMATION FOR THE USER FEXOFENADINE HYDROCHLORIDE 120 MG FILM-COATED TABLETS FEXOFENADINE HYDROCHLORIDE 180 MG FILM-COATED TABLETS Fexofenadine hydrochloride Read all of this leaflet carefully before you start using this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or pharmacist. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any of the side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet, (see section 4). In this leaflet: 1. What Fexofenadine hydrochloride is and what it is used for 2. What you need to know before you take Fexofenadine hydrochloride 3. How to take Fexofenadine hydrochloride 4. Possible side effects of Fexofenadine hydrochloride 5. How to store Fexofenadine hydrochloride 6. Contents of the pack and other information 1. WHAT FEXOFENADINE HYDROCHLORIDE IS AND WHAT IT IS USED FOR FEXOFENADINE HYDROCHLORIDE Contains fexofenadine hydrochloride which is an antihistamine. Only Fexofenadine hydrochloride 120 mg tablets is used in adults and adolescents of 12 years and older to relieve the symptoms that occur with hay fever (seasonal allergic rhinitis) such as sneezing, itchy, running or blocked nose and itchy, red and watery eye). Fexofenadine hydrochloride 180 mg tablets is used in adults and adolescents of 12 years and older to relieve the symptoms that occur with long term allergic skin reactions( chronic idiopathic urticaria) such as itching, swelling and rashes 2. -
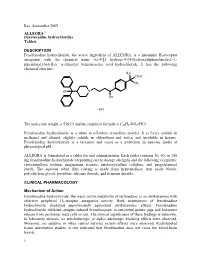
Fexofenadine Hydrochloride) Tablets
Rev. September 2005 ALLEGRA® (fexofenadine hydrochloride) Tablets DESCRIPTION Fexofenadine hydrochloride, the active ingredient of ALLEGRA, is a histamine H1-receptor antagonist with the chemical name (±)-4-[1 hydroxy-4-[4-(hydroxydiphenylmethyl)-1- piperidinyl]-butyl]-α, α-dimethyl benzeneacetic acid hydrochloride. It has the following chemical structure: The molecular weight is 538.13 and the empirical formula is C32H39NO4•HCl. Fexofenadine hydrochloride is a white to off-white crystalline powder. It is freely soluble in methanol and ethanol, slightly soluble in chloroform and water, and insoluble in hexane. Fexofenadine hydrochloride is a racemate and exists as a zwitterion in aqueous media at physiological pH. ALLEGRA is formulated as a tablet for oral administration. Each tablet contains 30, 60, or 180 mg fexofenadine hydrochloride (depending on the dosage strength) and the following excipients: croscarmellose sodium, magnesium stearate, microcrystalline cellulose, and pregelatinized starch. The aqueous tablet film coating is made from hypromellose, iron oxide blends, polyethylene glycol, povidone, silicone dioxide, and titanium dioxide. CLINICAL PHARMACOLOGY Mechanism of Action Fexofenadine hydrochloride, the major active metabolite of terfenadine, is an antihistamine with selective peripheral H1-receptor antagonist activity. Both enantiomers of fexofenadine hydrochloride displayed approximately equipotent antihistaminic effects. Fexofenadine hydrochloride inhibited antigen-induced bronchospasm in sensitized guinea pigs and histamine -

Prescribing Information for High-Dose Fexofenadine in the Management of Urticaria in Adults
1 Prescribing information for high-dose fexofenadine in the management of urticaria in adults This prescribing information document outlines the prescribing responsibilities between the specialist and GP. GPs are invited to participate. If the GP feels that such prescribing is outside their area of expertise or have clinical concerns about the safe management of the drug in primary care, then he or she is under no obligation to do so. In such an event, clinical responsibility for the patient’s health remains with the specialist. If a specialist asks the GP to prescribe, the GP should reply to this request as soon as practicable. Consultant details GP details Patient details Name: Name: Name: Address: Address: NHS Number: Email: Email: Date of birth: Contact number: Contact number: Contact: Introduction Fexofenadine is a second generation non-sedating H1-antihistamine used in the treatment of allergic disorders. Fexofenadine is a pharmacologically active metabolite of terfenadine. Licensed indication: In adults and children 12 years and older, the licensed dose is 180mg daily for the relief of symptoms associated with chronic idiopathic urticaria. Unlicensed indication (the focus of this document): As advised by Europeani and British Association of Dermatologistsii guidelines, doses of antihistamines up to four times the recommended daily dose may be used in the treatment of urticaria. Adult dosage and administration The licensed maximum recommended daily dose is 180mg once daily taken before a meal. Doses of up to four times the daily recommended dose have been used in the treatment of severe urticaria (unlicensed indication)i Available as: 30mg, 120mg and 180mg tablets Specialist responsibilities Provide patient/carer with relevant written information on the unlicensed use of high-dose fexofenadine and possible side-effects. -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -

Antihistamines in the Treatment of Chronic Urticaria I Jáuregui,1 M Ferrer,2 J Montoro,3 I Dávila,4 J Bartra,5 a Del Cuvillo,6 J Mullol,7 J Sastre,8 a Valero5
Antihistamines in the treatment of chronic urticaria I Jáuregui,1 M Ferrer,2 J Montoro,3 I Dávila,4 J Bartra,5 A del Cuvillo,6 J Mullol,7 J Sastre,8 A Valero5 1 Service of Allergy, Hospital de Basurto, Bilbao, Spain 2 Department of Allergology, Clínica Universitaria de Navarra, Pamplona, Spain 3 Allergy Unit, Hospital La Plana, Villarreal (Castellón), Spain 4 Service of Immunoallergy, Hospital Clínico, Salamanca, Spain 5 Allergy Unit, Service of Pneumology and Respiratory Allergy, Hospital Clínic (ICT), Barcelona, Spain 6 Clínica Dr. Lobatón, Cádiz, Spain 7 Rhinology Unit, ENT Service (ICEMEQ), Hospital Clínic, Barcelona, Spain 8 Service of Allergy, Fundación Jiménez Díaz, Madrid, Spain ■ Summary Chronic urticaria is highly prevalent in the general population, and while there are multiple treatments for the disorder, the results obtained are not completely satisfactory. The second-generation H1 antihistamines remain the symptomatic treatment option of choice. Depending on the different pharmacokinetics and H1 receptor affi nity of each drug substance, different concentrations in skin can be expected, together with different effi cacy in relation to the histamine-induced wheal inhibition test - though this does not necessarily have repercussions upon clinical response. The antiinfl ammatory properties of the H1 antihistamines could be of relevance in chronic urticaria, though it is not clear to what degree they infl uence the fi nal therapeutic result. Before moving on to another therapeutic level, the advisability of antihistamine dose escalation should be considered, involving increments even above those approved in the Summary of Product Characteristics. Physical urticaria, when manifesting isolatedly, tends to respond well to H1 antihistamines, with the exception of genuine solar urticaria and delayed pressure urticaria. -

Drugs Affectin the Autonomic Nervous System
Fundamentals of Medical Pharmacology Paterson Public Schools Written by Néstor Collazo, Ph.D. Jonathan Hodges, M.D. Tatiana Mikhaelovsky, M.D. for Health and Related Professions (H.A.R.P.) Academy March 2007 Course Description This fourth year course is designed to give students in the Health and Related Professions (H.A.R.P.) Academy a general and coherent explanation of the science of pharmacology in terms of its basic concepts and principles. Students will learn the properties and interactions between chemical agents (drugs) and living organisms for the rational and safe use of drugs in the control, prevention, and therapy of human disease. The emphasis will be on the fundamental concepts as they apply to the actions of most prototype drugs. In order to exemplify important underlying principles, many of the agents in current use will be singled out for fuller discussion. The course will include the following topics: ¾ The History of Pharmacology ¾ Terminology Used in Pharmacology ¾ Drug Action on Living Organisms ¾ Principles of Pharmacokinetics ¾ Dose-Response Relationships ¾ Time-Response Relationships ¾ Human Variability: Factors that will modify effects of drugs on individuals ¾ Effects of Drugs Attributable to Varying Modes of Administration ¾ Drug Toxicity ¾ Pharmacologic Aspects of Drug Abuse and Drug Dependence Pre-requisites Students must have completed successfully the following courses: Biology, Chemistry, Anatomy and Physiology, Algebra I and II Credits: 5 credits Basic Principles of Drug Action Introduction to Pharmacology a. Basic Mechanisms of Drug Actions b. Dose-response relationships c. Drug absorption d. Biotransformation of Drugs e. Pharmacokinetics f. Factors Affecting Drug Distribution g. Drug Allergy and Pharmacogenetics h. -

A Study of Effect of a Single Dose of Second Generation Antihistaminics on Cognitive and Psychomotor Function in Healthy Human Volunteers
International Journal of Basic & Clinical Pharmacology Saxena K et al. Int J Basic Clin Pharmacol. 2020 Dec;9(12):1836-1843 http:// www.ijbcp.com pISSN 2319-2003 | eISSN 2279-0780 DOI: https://dx.doi.org/10.18203/2319-2003.ijbcp20205120 Original Research Article A study of effect of a single dose of second generation antihistaminics on cognitive and psychomotor function in healthy human volunteers Kirti Saxena*, Sachendra K. Srivastava, Chaitali Mehta Department of Pharmacology, Surat Municipal Institute of Medical Education and Research, Surat, Gujarat, India Received: 26 September 2020 Revised: 01 November 2020 Accepted: 02 November 2020 *Correspondence: Dr. Kirti Saxena, Email: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Objective of the study was to assess whether second generation antihistaminic alter psychomotor and cognitive function in comparison with promethazine (marked sedation; altered psychomotor and cognitive impairment). Methods: It was a single blind prospective study. Seventy five healthy human volunteers were registered, divided in five groups. These groups have received placebo, promethazine 25 mg, cetirizine 10 mg, fexofenadine 120 mg and loratadine 10 mg. Cognitive and psychomotor functions were assessed pretreatment and 60 minutes after single dose of drug(post treatment)by using a battery of standard tests (e.g. PST-Perceptual speed test, BVRT-Benton visual retention test,SSS- Stanford Sleepiness Scale, FTT-Finger tapping test etc.). -

Antihistamines and Driving-Related Behavior: a Review of the Evidence for Impairment by First- Versus Second-Generation H1-Antagonists
DOT HS 809 714 May 2004 Technical Report Documentation Page 1. Report No. 2. Government Accession No. 3. Recipient's Catalog No. DOT HS 809 714 4. Title and Subtitle 5. Report Date ANTIHISTAMINES AND DRIVING-RELATED BEHAVIOR: May 2004 A REVIEW OF THE EVIDENCE FOR IMPAIRMENT 6. Performing Organization Code 7. Author(s) 8. Performing Organization Report No. Herbert Moskowitz, Ph.D. and Candace Jeavons Wilkinson, Ph.D. 9. Performing Organization Name and Address 10. Work Unit No. (TRAIS) Herbert Moskowitz, Ph.D., Inc. 11. Contract or Grant No. 4138 Royal Crest Place, Encino, CA 91436 12. Sponsoring Agency Name and Address’ 13. Type of Report and Period Covered U.S. Department of Transportation Final Report National Highway Traffic Safety Administration Period: September 2000-February 2003 400 Seventh Street, S.W Washington, D.C. 20590 14. Sponsoring Agency Code 15. Supplementary Notes 16. Abstract A review of the scientific literature concerning the effects of antihistamines on driving-related skills was conducted. After reviewing all pertinent publications from 1998 and earlier, a total of 130 publications were found to meet criteria for inclusion in the data summaries. A data base was created with study results being indexed, and summarized, by specific drug, dose, dosing schedule (i.e., single versus repeated) and H1-antagonist generation as well as by behavioral area or subjective measure. For each H1-antagonist generation, five drugs were evaluated: chlorpheniramine, clemastine, diphenhydramine, hydroxyzine and tripolidine for the 1st-generation, and astemizole, cetirizine, fexofenadine, loratadine and terfenadine for the 2nd-generation. It was concluded that: 1. There is some slight, but ambiguous, evidence from epidemiological studies of a connection between antihistamine use and traffic collision rates. -

FEXOFENADINE HYDROCHLORIDE - Fexofenadine Hydrochloride Tablet, Film Coated Magno-Humphries Labs, Inc
FEXOFENADINE HYDROCHLORIDE - fexofenadine hydrochloride tablet, film coated Magno-Humphries Labs, Inc. ---------- Active ingredient(in each tablet) Fexofenadine HCl USP, 180 mg Purpose Antihistamine Uses temporarily relieves these symptoms due to hay fever or other upper respiratory allergies: runny nose sneezing itchy, watery eyes itching of the nose or throat Warnings Do not use if you have ever had an allergic reaction to this product or any of its ingredients Ask a doctor before use if you have kidney disease. Your doctor should determine if you need a different dose. When using this product do not take more than directed do not take at the same time as aluminium or magnesium antacids do not take with fruit juices (see directions) Stop use and ask doctor if an allergic reaction to this product occurs. Seek medical help right away. If pregnant or breast-feeding ask a health professional before use. Keep out of reach of children In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away. Directions adults and children 12 years of age and 1 tablet with water a day ; do not take more than 1 tablet in 24 over hours children under 12 years of age do not use adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor Other information store between 20°and 25°C (68°and 77°F) protect from excessive moisture and light Inactive ingredients anhydrous lactose, colloidal silicon dioxide, corn starch,croscarmellose sodium, hypromellose, lactose monohydrate, polyethylene glycol -

Medications to Avoid in Long QT Syndrome
Jackie Crawford Cardiac Inherited Disease Co-ordinator C/- Paediatric Cardiology; Level 3 Starship; Auckland City Hospital Private Bag 92024; Auckland Cardiac Inherited Disease Registry Phone: (09) 3074949 ext 23634 www.cidg.org Fax: (09) 6309877 Email: [email protected] Medications to be avoided, or requiring special caution, in people with Long QT syndrome This list includes medications which prolong the QT interval and is meant as a guide for people with Long QT syndrome, or acquired long QT interval from heart muscle disease, and their parents or guardians. It should not be seen as all inclusive. Those prescribing any medication to someone with Long QT syndrome should always check the drug specifications and contra-indications. The list has been compiled by review of publications and/or drug advice sheets provided with medications. Check also www.SADS.org and www.Torsades .org. Antibiotics Erythromycin, Clarithromycin, Gatifloxacin, levofloxacin, Moxifloxacin Sulfamethoxazole-trimethoprim (Septrin/Bactrim), Spiramycin, Pentamidine Antihistamines Terfenadine, Astemizole, Diphenhydramine, (These are particularly to be avoided (even in normal subjects) in combination with Erythromycin or grapefruit juice or the antifungals ketoconazole, miconazole, fluconazole or itraconazole) [Antihistamines that may be used safely are loratidine, cetirizine and fexofenadine, and phenergan] Appetite suppressants Fenfluramine, phentermine, Sibutramine Asthma treatments The Beta-2 agonists (e.g. Terbutaline, Salbutamol, Salmeterol) both work against -

Medications to Avoid Before Skin Testing
PLEASE STOP ANTIHISTAMINES 5 DAYS PRIOR TO NEW PATIENT APPOINTMENTS OR ALLERGY SKIN TESTING *Do not stop asthma medications or any other medications that do not contain antihistamine! **If you have major hives or swelling, do not stop your antihistamines. ***Please call us if you have questions about any of your medications interfering with skin testing. ****Do not use oil, cream or lotion on the back or arms for 24 hours prior to skin testing. COMMON MEDICATIONS CONTAINING ANTIHISTAMINES • Actifed (chlorpheniramine) • Elavil (amitriptyline) • Advil PM, Advil Allergy • Excedrin PM • Alavert/Claritin (loratadine) • Fexofenadine (Allegra) • Allegra (fexofenadine) • Hydroxyzine (Atarax, Vistaril) • Alka Seltzer P.M. • Imipramine (Tofranil) • Amitriptyline (Elavil) • Levocetirizine (Xyzal) • Antivert (Meclizine) • Loratadine (Claritin) • Astelin Nasal Spray (azelastine) • Meclizine (Antivert, Bonine) • Astepro Nasal Spray (azelastine) • Norpramine (desipramine) • Atarax (hydroxyzine) • Nortriptyline (Pamelor) • Azelastine nose spray (Astepro, Astelin) • Nyquil • Benadryl (diphenhydramine) • Nytol • Bonine (meclizine) • Olopatadine (Patanase nasal spray) • Cetirizine (Zyrtec) • Pamelor (nortriptyline) • Chlorpheniramine (Chlor-Trimeton, Actifed, • Patanase Nasal Spray (olopatadine) Tussionex) • PBZ (pyribenzamine) • Chlor-Trimeton (chlorpheniramine) • Pediacare • Clarinex (desloratadine) • Periactin (Cyproheptadine) • Claritin (loratadine) • Phenergan (promethazine) • Clemastine (Tavist) • Promethazine (Phenergan) • Cogentin (for Parkinson's