Setting Evolutionarybased Conservation Priorities for A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

St. Kitts Final Report
ReefFix: An Integrated Coastal Zone Management (ICZM) Ecosystem Services Valuation and Capacity Building Project for the Caribbean ST. KITTS AND NEVIS FIRST DRAFT REPORT JUNE 2013 PREPARED BY PATRICK I. WILLIAMS CONSULTANT CLEVERLY HILL SANDY POINT ST. KITTS PHONE: 1 (869) 765-3988 E-MAIL: [email protected] 1 2 TABLE OF CONTENTS Page No. Table of Contents 3 List of Figures 6 List of Tables 6 Glossary of Terms 7 Acronyms 10 Executive Summary 12 Part 1: Situational analysis 15 1.1 Introduction 15 1.2 Physical attributes 16 1.2.1 Location 16 1.2.2 Area 16 1.2.3 Physical landscape 16 1.2.4 Coastal zone management 17 1.2.5 Vulnerability of coastal transportation system 19 1.2.6 Climate 19 1.3 Socio-economic context 20 1.3.1 Population 20 1.3.2 General economy 20 1.3.3 Poverty 22 1.4 Policy frameworks of relevance to marine resource protection and management in St. Kitts and Nevis 23 1.4.1 National Environmental Action Plan (NEAP) 23 1.4.2 National Physical Development Plan (2006) 23 1.4.3 National Environmental Management Strategy (NEMS) 23 1.4.4 National Biodiversity Strategy and Action Plan (NABSAP) 26 1.4.5 Medium Term Economic Strategy Paper (MTESP) 26 1.5 Legislative instruments of relevance to marine protection and management in St. Kitts and Nevis 27 1.5.1 Development Control and Planning Act (DCPA), 2000 27 1.5.2 National Conservation and Environmental Protection Act (NCEPA), 1987 27 1.5.3 Public Health Act (1969) 28 1.5.4 Solid Waste Management Corporation Act (1996) 29 1.5.5 Water Courses and Water Works Ordinance (Cap. -

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Pseudosiderastrea Formosa Sp. Nov. (Cnidaria: Anthozoa: Scleractinia)
Zoological Studies 51(1): 93-98 (2012) Pseudosiderastrea formosa sp. nov. (Cnidaria: Anthozoa: Scleractinia) a New Coral Species Endemic to Taiwan Michel Pichon1, Yao-Yang Chuang2,3, and Chaolun Allen Chen2,3,4,* 1Museum of Tropical Queensland, 70-102 Flinders Street, Townsville 4810, Australia 2Biodiversity Research Center, Academia Sinica, Nangang, Taipei 115, Taiwan 3Institute of Oceanography, National Taiwan Univ., Taipei 106, Taiwan 4Institute of Life Science, National Taitung Univ., Taitung 904, Taiwan (Accepted September 1, 2011) Michel Pichon, Yao-Yang Chuang, and Chaolun Allen Chen (2012) Pseudosiderastrea formosa sp. nov. (Cnidaria: Anthozoa: Scleractinia) a new coral species endemic to Taiwan. Zoological Studies 51(1): 93-98. Pseudosiderastrea formosa sp. nov. is a new siderastreid scleractinian coral collected in several localities in Taiwan. It lives on rocky substrates where it forms encrusting colonies. Results of morphological observations and molecular genetic analyses are presented. The new species is described and compared to P. tayamai and Siderastrea savignyana, and its morphological and phylogenic affinities are discussed. http://zoolstud.sinica.edu.tw/Journals/51.1/93.pdf Key words: Pseudosiderastrea formosa sp. nov., New species, Scleractinia, Siderastreid, Western Pacific Ocean. A siderastreid scleractinian coral was Pseudosiderastrea, described as P. formosa sp. collected from several localities around Taiwan nov. and nearby islands, where it is relatively rare. The specimens present some morphological similarities with Pseudosiderastrea tayamai Yabe MATERIAL AND METHODS and Sugiyama, 1935, the only species hitherto known from that genus, and with Siderastrea Specimens were collected by scuba diving at savignyana Milne Edwards and Haime, 1849, Wanlitung (21°59'48"N, 120°42'10"E) and the outlet which is the sole representative in the Indian of the 3rd nuclear power plant (21°55'51.38"N, Ocean of the genus Siderastrea de Blainville, 120°44'46.82"E) on the southeastern coast 1830. -

Guide to Some Harvested Aquarium Corals Version 1.3
Guide to some harvested aquarium corals Version 1.3 ( )1 Large septal Guide to some harvested aquarium teeth corals Version 1.3 Septa Authors Morgan Pratchett & Russell Kelley, May 2020 ARC Centre of Excellence for Coral Reef Studies Septa James Cook University Townsville, Queensland 4811 Australia Contents • Overview in life… p3 • Overview of skeletons… p4 • Cynarina lacrymalis p5 • Acanthophyllia deshayesiana p6 • Homophyllia australis p7 • Micromussa pacifica p8 • Unidentified Lobophylliid p9 • Lobophyllia vitiensis p10 • Catalaphyllia jardinei p11 • Trachyphyllia geoffroyi p12 Mouth • Heterocyathus aequicostatus & Heteropsammia cochlea p13 Small • Cycloseris spp. p14 septal • Diaseris spp. p15 teeth • Truncatoflabellum sp. p16 Oral disk Meandering valley Bibliography p17 Acknowledgements FRDC (Project 2014-029) Image support: Russell Kelley, Cairns Marine, Ultra Coral, JEN Veron, Jake Adams, Roberto Arrigioni ( )2 Small septal teeth Guide to some commonly harvested aquarium corals - Version 1.3 Overview in life… SOLID DISKS WITH FLESHY POLYPS AND PROMINENT SEPTAL TEETH Cynarina p5 Acanthophyllia p6 Homophyllia p7 Micromussa p8 Unidentified Lobophylliid p9 5cm disc, 1-2cm deep, large, thick, white 5-10cm disc at top of 10cm curved horn. Tissue 5cm disc, 1-2cm deep. Cycles of septa strongly <5cm disc, 1-2cm deep. Cycles of septa slightly septal teeth usually visible through tissue. In unequal. Large, tall teeth at inner marigns of primary unequal. Teeth of primary septa less large / tall at conceals septa. In Australia usually brown with inner margins. Australia usually translucent green or red. blue / green trim. septa. In Australia traded specimens are typically variegated green / red / orange. 2-3cm disc, 1-2cm deep. Undescribed species traded as Homophyllia australis in West Australia and Northern Territory but now recognised as distinct on genetic and morphological grounds. -

Stewardship Action Plan a Statement of Operational Standards and Climate Change Contingency Planning
STEWARDSHIP ACTION PLAN A statement of operational standards and climate change contingency planning For Pro-vision Reef members who are license holders in the: enter Queensland Marine Aquarium Fish Fishery and the Proudly supported by: Queensland Coral Fishery and the Coral Sea Fishery (Aquarium Collection) EFF E CTIVE 2009 Navigation: Foreword | Preface | Contents | Part 1 | 2 | 3 | 4 | 5 | 6 | 7 | Bibliography | Attachment 1 | 2 Cover Photo: Blueface Angelfish (Pomacanthus xanthometopon). Photo by Fenton Walsh, Northern Barrier Marinelife, Cairns QLD Australia. Copyright © Pro-vision Reef Inc 2009 This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from Pro-vision Reef Inc. Requests and inquiries concerning reproduction and rights should be addressed to: President Pro-vision Reef Inc Email: [email protected] This publication is available online at www.pro-visionreef.org First published 2009 by: Pro-vision Reef Inc ABN: 57 442 161 812 PO Box 5N Cairns North QLD Australia 4870 National Library of Australia Cataloguing in Publication entry: Pro-vision Reef: Stewardship Action Plan. A Statement of Operational Standards and Climate Change Contingency Planning. ISBN: 978-0-646-51212-9 Author: Ryan Donnelly, Strategic Projects Manager, Cairns Marine Pty Ltd Design: Andreas Wagner, www.coolplanetdesign.com.au The author acknowledges the guidance and input from the Pro-vision Reef committee: Lyle Squire Jnr, Fenton Walsh, Don Gilson and Ros Paterson; and the support and contribution from Margie Atkinson from the Great Barrier Reef Marine Park Authority; Tara Smith and Brigid Kerrigan from Queensland Primary Industries & Fisheries; and Josh Davis from the Australian Fisheries Management Authority. -

Icrs2012 17D 6
Proceedings of the 12th International Coral Reef Symposium, Cairns, Australia, 9-13 July 2012 17D Managing bleached coral reefs First Quantitative Assessment of Coral Bleaching on Indonesian Reefs Syafyudin Yusuf1, Jamaluddin Jompa1,2 1Center for Coral Reef Research, Hasanuddin University Makassar Indonesia 2Marine Coastal and Small Island Research and Development of Hasanuddin University contact : [email protected] ABSTRACT Many reefs around the world have experienced repeated disturbances, particularly severe coral bleaching followed by coral mortality in the past decades, however, there have been few quantitative assessments of the impact of bleaching on Indonesian reefs and none published from Sulawesi. A four-year monitoring program (2007-2010) of coral reefs in the Spermonde and of Buton reefs in Sulawesi indicated that coral bleaching only occurred in the years 2009-2010. Here we provide the first report of bleaching prevalence among coral colonies and genera based on 142 quadrats (50 cm x 50 cm) and 87 digital photos in Spermonde, and 257 quadrats and 96 digital photos of bleached colonies in Buton reefs. The results show that the scleractinian families most susceptible to bleaching were the Acroporidae, Faviidae, Poritidae and Pocilloporoidae, and the Alcyonacea were also affected. The genera most susceptible to bleaching were the Acropora, Diploastrea, Favia, Favites, Goniopora, Porites, Pocillopora and Sinularia. The genera most resistant to bleaching were Turbinaria, Pachyseris, Symphyllia, and Heliofungia actiniformis. Sixty percent of species were susceptible to bleaching in Spermonde reef and 58% in Buton reefs. These bleaching events decreased live coral cover by 12.45% in Spermonde Reef between 2009-2010. Fishermen on these reefs first saw the bleaching in May; other respondents saw bleaching in June and July 2010. -

Resurrecting a Subgenus to Genus: Molecular Phylogeny of Euphyllia and Fimbriaphyllia (Order Scleractinia; Family Euphylliidae; Clade V)
Resurrecting a subgenus to genus: molecular phylogeny of Euphyllia and Fimbriaphyllia (order Scleractinia; family Euphylliidae; clade V) Katrina S. Luzon1,2,3,*, Mei-Fang Lin4,5,6,*, Ma. Carmen A. Ablan Lagman1,7, Wilfredo Roehl Y. Licuanan1,2,3 and Chaolun Allen Chen4,8,9,* 1 Biology Department, De La Salle University, Manila, Philippines 2 Shields Ocean Research (SHORE) Center, De La Salle University, Manila, Philippines 3 The Marine Science Institute, University of the Philippines, Quezon City, Philippines 4 Biodiversity Research Center, Academia Sinica, Taipei, Taiwan 5 Department of Molecular and Cell Biology, James Cook University, Townsville, Australia 6 Evolutionary Neurobiology Unit, Okinawa Institute of Science and Technology Graduate University, Okinawa, Japan 7 Center for Natural Sciences and Environmental Research (CENSER), De La Salle University, Manila, Philippines 8 Taiwan International Graduate Program-Biodiversity, Academia Sinica, Taipei, Taiwan 9 Institute of Oceanography, National Taiwan University, Taipei, Taiwan * These authors contributed equally to this work. ABSTRACT Background. The corallum is crucial in building coral reefs and in diagnosing systematic relationships in the order Scleractinia. However, molecular phylogenetic analyses revealed a paraphyly in a majority of traditional families and genera among Scleractinia showing that other biological attributes of the coral, such as polyp morphology and reproductive traits, are underutilized. Among scleractinian genera, the Euphyllia, with nine nominal species in the Indo-Pacific region, is one of the groups Submitted 30 May 2017 that await phylogenetic resolution. Multiple genetic markers were used to construct Accepted 31 October 2017 Published 4 December 2017 the phylogeny of six Euphyllia species, namely E. ancora, E. divisa, E. -

Poster Presentations Anthropogenic Impacts Interacting Influence of Low
Poster Presentations Anthropogenic Impacts Interacting influence of low salinity and nutrient pulses on the growth of bloom-forming Ulva compressa Guidone, M.; Steele, L. Sacred Heart University, Fairfield, CT 06825. [email protected] For the eastern US, it is predicted that climate change will increase the frequency of severe rainstorms, inundating coastal areas with pulses of freshwater that will reduce salinity but also temparily increase nutrients through sewage overflow and storm runoff. In an effort to predict how this may influence the frequency and severity of macroalgal blooms, this study examined the interacting effect of salinity and nutrient supply on bloom-forming Ulva compressa. In laboratory experiments with constant nutrient supply, U. compressa showed decreased growth at low salinities; however, this decrease was not detectable until the fifth day of treatment. Moreover, U. compressa demonstrated an extraordinary tolerance for freshwater, surviving for 48 hours without nutrients at 0 PSU. When exposed to pulses of freshwater (0 PSU) and varying nutrient levels (none, low, high) lasting either 0.5, 4, or 8 hours, U. compressa growth was negatively impacted in the treatment without nutrients and positively impacted by the low and high nutrient treatments. Furthermore, thalli in the high nutrient treatment showed increased growth with increased pulse time. These findings, combined with observations of U. compressa’s tolerance for high temperatures, suggest Ulva blooms will not decrease in frequency or severity with a change in precipitation patterns. Additional information: a) Faculty b) Poster c) Anthropogenic impacts; Community ecology d) No Long Term Effects of Anthropogenic Influences on Marine Benthic Macrofauna Adjacent to McMurdo Station, Antarctica Smith, S.1; Montagna, P.; Palmer, T.; Hyde, L. -

BIOT Field Report
©2015 Khaled bin Sultan Living Oceans Foundation. All Rights Reserved. Science Without Borders®. All research was completed under: British Indian Ocean Territory, The immigration Ordinance 2006, Permit for Visit. Dated 10th April, 2015, issued by Tom Moody, Administrator. This report was developed as one component of the Global Reef Expedition: BIOT research project. Citation: Global Reef Expedition: British Indian Ocean Territory. Field Report 19. Bruckner, A.W. (2015). Khaled bin Sultan Living Oceans Foundation, Annapolis, MD. pp 36. The Khaled bin Sultan Living Oceans Foundation (KSLOF) was incorporated in California as a 501(c)(3), public benefit, Private Operating Foundation in September 2000. The Living Oceans Foundation is dedicated to providing science-based solutions to protect and restore ocean health. For more information, visit http://www.lof.org and https://www.facebook.com/livingoceansfoundation Twitter: https://twitter.com/LivingOceansFdn Khaled bin Sultan Living Oceans Foundation 130 Severn Avenue Annapolis, MD, 21403, USA [email protected] Executive Director Philip G. Renaud Chief Scientist Andrew W. Bruckner, Ph.D. Images by Andrew Bruckner, unless noted. Maps completed by Alex Dempsey, Jeremy Kerr and Steve Saul Fish observations compiled by Georgia Coward and Badi Samaniego Front cover: Eagle Island. Photo by Ken Marks. Back cover: A shallow reef off Salomon Atoll. The reef is carpeted in leather corals and a bleached anemone, Heteractis magnifica, is visible in the fore ground. A school of giant trevally, Caranx ignobilis, pass over the reef. Photo by Phil Renaud. Executive Summary Between 7 March 2015 and 3 May 2015, the Khaled bin Sultan Living Oceans Foundation conducted two coral reef research missions as components of our Global Reef Expedition (GRE) program. -

Scleractinia Fauna of Taiwan I
Scleractinia Fauna of Taiwan I. The Complex Group 台灣石珊瑚誌 I. 複雜類群 Chang-feng Dai and Sharon Horng Institute of Oceanography, National Taiwan University Published by National Taiwan University, No.1, Sec. 4, Roosevelt Rd., Taipei, Taiwan Table of Contents Scleractinia Fauna of Taiwan ................................................................................................1 General Introduction ........................................................................................................1 Historical Review .............................................................................................................1 Basics for Coral Taxonomy ..............................................................................................4 Taxonomic Framework and Phylogeny ........................................................................... 9 Family Acroporidae ............................................................................................................ 15 Montipora ...................................................................................................................... 17 Acropora ........................................................................................................................ 47 Anacropora .................................................................................................................... 95 Isopora ...........................................................................................................................96 Astreopora ......................................................................................................................99 -

Jan 2021 London Zoo Stocklist.Pdf (596.63
ZSL London Zoo - January 2021 stocklist Status at 01.01.2021 m f unk Invertebrata Aurelia aurita * Moon jellyfish 0 0 150 Pachyclavularia violacea * Purple star coral 0 0 1 Tubipora musica * Organ-pipe coral 0 0 2 Pinnigorgia sp. * Sea fan 0 0 20 Sarcophyton sp. * Leathery soft coral 0 0 5 Sinularia sp. * Leathery soft coral 0 0 18 Sinularia dura * Cabbage leather coral 0 0 4 Sinularia polydactyla * Many-fingered leather coral 0 0 3 Xenia sp. * Yellow star coral 0 0 1 Heliopora coerulea * Blue coral 0 0 12 Entacmaea quadricolor Bladdertipped anemone 0 0 1 Epicystis sp. * Speckled anemone 0 0 1 Phymanthus crucifer * Red beaded anemone 0 0 11 Heteractis sp. * Elegant armed anemone 0 0 1 Stichodactyla tapetum Mini carpet anemone 0 0 1 Discosoma sp. * Umbrella false coral 0 0 21 Rhodactis sp. * Mushroom coral 0 0 8 Ricordea sp. * Emerald false coral 0 0 19 Acropora sp. * Staghorn coral 0 0 115 Acropora humilis * Staghorn coral 0 0 1 Acropora yongei * Staghorn coral 0 0 2 Montipora sp. * Montipora coral 0 0 5 Montipora capricornis * Coral 0 0 5 Montipora confusa * Encrusting coral 0 0 22 Montipora danae * Coral 0 0 23 Montipora digitata * Finger coral 0 0 6 Montipora foliosa * Hard coral 0 0 10 Montipora hodgsoni * Coral 0 0 2 Pocillopora sp. * Cauliflower coral 0 0 27 Seriatopora hystrix * Bird nest coral 0 0 8 Stylophora sp. * Cauliflower coral 0 0 1 Stylophora pistillata * Pink cauliflower coral 0 0 23 Catalaphyllia jardinei * Elegance coral 0 0 4 Euphyllia ancora * Crescent coral 0 0 4 Euphyllia glabrescens * Joker's cap coral 0 0 2 Euphyllia paradivisa * Branching frog spawn 0 0 3 Euphyllia paraancora * Branching hammer coral 0 0 3 Euphyllia yaeyamaensis * Crescent coral 0 0 4 Plerogyra sinuosa * Bubble coral 0 0 1 Duncanopsammia axifuga + Coral 0 0 2 Tubastraea sp. -
Jan 2021 ZSL Stocklist.Pdf (699.26
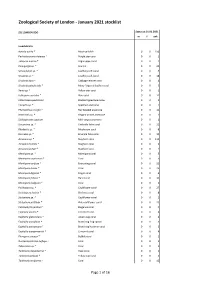
Zoological Society of London - January 2021 stocklist ZSL LONDON ZOO Status at 01.01.2021 m f unk Invertebrata Aurelia aurita * Moon jellyfish 0 0 150 Pachyclavularia violacea * Purple star coral 0 0 1 Tubipora musica * Organ-pipe coral 0 0 2 Pinnigorgia sp. * Sea fan 0 0 20 Sarcophyton sp. * Leathery soft coral 0 0 5 Sinularia sp. * Leathery soft coral 0 0 18 Sinularia dura * Cabbage leather coral 0 0 4 Sinularia polydactyla * Many-fingered leather coral 0 0 3 Xenia sp. * Yellow star coral 0 0 1 Heliopora coerulea * Blue coral 0 0 12 Entacmaea quadricolor Bladdertipped anemone 0 0 1 Epicystis sp. * Speckled anemone 0 0 1 Phymanthus crucifer * Red beaded anemone 0 0 11 Heteractis sp. * Elegant armed anemone 0 0 1 Stichodactyla tapetum Mini carpet anemone 0 0 1 Discosoma sp. * Umbrella false coral 0 0 21 Rhodactis sp. * Mushroom coral 0 0 8 Ricordea sp. * Emerald false coral 0 0 19 Acropora sp. * Staghorn coral 0 0 115 Acropora humilis * Staghorn coral 0 0 1 Acropora yongei * Staghorn coral 0 0 2 Montipora sp. * Montipora coral 0 0 5 Montipora capricornis * Coral 0 0 5 Montipora confusa * Encrusting coral 0 0 22 Montipora danae * Coral 0 0 23 Montipora digitata * Finger coral 0 0 6 Montipora foliosa * Hard coral 0 0 10 Montipora hodgsoni * Coral 0 0 2 Pocillopora sp. * Cauliflower coral 0 0 27 Seriatopora hystrix * Bird nest coral 0 0 8 Stylophora sp. * Cauliflower coral 0 0 1 Stylophora pistillata * Pink cauliflower coral 0 0 23 Catalaphyllia jardinei * Elegance coral 0 0 4 Euphyllia ancora * Crescent coral 0 0 4 Euphyllia glabrescens * Joker's cap coral 0 0 2 Euphyllia paradivisa * Branching frog spawn 0 0 3 Euphyllia paraancora * Branching hammer coral 0 0 3 Euphyllia yaeyamaensis * Crescent coral 0 0 4 Plerogyra sinuosa * Bubble coral 0 0 1 Duncanopsammia axifuga + Coral 0 0 2 Tubastraea sp.