Thermal Radiative Ignition of Liquid Fuels by a CO₂ Laser
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Schlieren Imaging
Traldi et al. EPJ Techniques and Instrumentation (2018) 5:4 https://doi.org/10.1140/epjti/s40485-018-0045-1 RESEARCH ARTICLE Open Access Schlieren imaging: a powerful tool for atmospheric plasma diagnostic Enrico Traldi1, Marco Boselli1,2, Emanuele Simoncelli1, Augusto Stancampiano3, Matteo Gherardi1,2, Vittorio Colombo1,2* and Gary S. Settles4* * Correspondence: vittorio. [email protected]; [email protected] Abstract 1Department of Industrial Engineering, Alma Mater Studiorum Schlieren imaging has been widely used in science and technology to investigate – Università di Bologna, Viale del phenomena occurring in transparent media. In particular, it has proven to be a Risorgimento 2, 40136 Bologna, Italy powerful tool in fundamental studies and process optimization for atmospheric 4FloViz Inc., Port Matilda, PA 16870, USA pressure plasma diagnostics, providing qualitative and (in some cases) also Full list of author information is quantitative information on the fluid-dynamic characteristics of plasmas generated available at the end of the article by many different types of sources. However, obtaining significant and reliable results by schlieren imaging can be challenging, especially when considering the variety of geometries and applications of atmospheric pressure plasma sources. Therefore, it is necessary to adopt solutions that can address the specific issues of different plasma-assisted processes. In this paper, an overview on the use of the schlieren imaging technique for atmospheric pressure plasma characterization is presented. In the first part, the physical principles behind this technique and the different setups that can be adopted to perform it are presented. In the second part, examples of schlieren imaging applied to different kinds of atmospheric pressure plasmas (non-equilibrium plasma jets, plasma actuators for flow control and thermal plasma sources) are presented, showing how it was used to characterize the fluid-dynamic behavior of plasma-assisted processes and reporting best practices in performing this diagnostic technique. -

Introduction to Shadowgraph and Schlieren Imaging Andrew Davidhazy
Rochester Institute of Technology RIT Scholar Works Articles 2006 Introduction to shadowgraph and schlieren imaging Andrew Davidhazy Follow this and additional works at: http://scholarworks.rit.edu/article Recommended Citation Davidhazy, Andrew, "Introduction to shadowgraph and schlieren imaging" (2006). Accessed from http://scholarworks.rit.edu/article/478 This Technical Report is brought to you for free and open access by RIT Scholar Works. It has been accepted for inclusion in Articles by an authorized administrator of RIT Scholar Works. For more information, please contact [email protected]. INTRODUCTION TO SHADOHGRAPH AND SCHLIEREN IMAGING Andrew Davidhazy Rochester In.titute of Technology Imaging and Photoqraphic Technology Schlieren photography is not new. It's a technique peripherally developed by early astronomers and glass maker•. It is a word that has it's origin in the german word "schliereN or streaks, caused by inhomogeneous areas in glass. A variety of methods were used to detect these .chliere, with some of them probably closely related to the technique. which we are about to examine. There are three basic types of - SI-tAbOIJ612APHS optical probing systems each with intrinsic merits and limitations. -SCHLIE'REN These are shadowqraphs, schlieren images, and interferograms. The5e are -UffiRffRD6I2AHS lis~ed in order of increasing complexi~y and pos5ible varia~ions. In ~he mid 1800's, Leon Foucault developed a test ~ha~ bears his name for examining the figure or curvature of as~ronomical mirrors. Worker5 testing mirror.~i~n the Foucault ~est were well aware of, and took great pains to minimize, the disturbing effects of density gradients present between the light FOUCAULT source, the mirror and the "knife edge". -

Aeronautical Laboratories Institute
GRADUATEAERONAUTICAL LABORATORIES CALIFORNIAINSTITUTE of TECHNOLOGY Pasadena, California 91125 The Supersonic Hydrogen-Fluorine Combustion Facility Design Review Report Version 4.0 by Jeffery Hall and Pad Dimotakis GALCIT Internal Report 30 August 1989 Acknowledgements This development of the facility to be described below is the outgrowth of a design, theoretical and experimental effort at GALCIT that began over ten years ago to study high Reynolds number turbulent mixing under Air Force Office of Scientific Research sponsor- ship. A large number of people have contributed to the development of this facility, which has been realized as a result of their contributions and the long-term support by AFOSR, with Dr. Julian Tishkoff the current program manager, which we gratefully acknowledge*. The first phase of that effort, which involved the study and behavior of subsonic shear layers, was realized in the subsonic shear layer facility which was designed and fabricated with the aid and contributions of several people. That design was modeled after the smaller subsonic facility built by Wallace (1981) under the supervision of Garry Brown, Professor in Aeronautics at the time and presently director of ARL in Australia. The GALCIT subsonic facility design was begun by r Brian Cantwell, Research Fellow in Aeronautics at the time, presently Professor at Stanford, in collaboration with and under the guidance of Garry Brown. It was completed and used as part of the P11.D. thesis research of M. Godfrey Mungal, Graduate Research Assistant and subsequently Research Fellow in Aeronautics, presently Assistant Professor at Stanford, under the guidance of H. W. L'iepmann, Director of GALCIT at the time and presently Professor Emer- itus in Aeronautics, and P. -

Utvrđivanje Izvornika Analognih I Digitalnih Fotografija
Filozofski fakultet Hrvoje Gržina UTVRĐIVANJE IZVORNIKA ANALOGNIH I DIGITALNIH FOTOGRAFIJA DOKTORSKI RAD Zagreb, 2017. Faculty of Humanities and Social Sciences Hrvoje Gržina IDENTIFYING ORIGINALS OF ANALOG AND DIGITAL PHOTOGRAPHS DOCTORAL THESIS Zagreb, 2017 Filozofski fakultet Hrvoje Gržina UTVRĐIVANJE IZVORNIKA ANALOGNIH I DIGITALNIH FOTOGRAFIJA DOKTORSKI RAD Mentor: dr. sc. Hrvoje Stančić, izv. prof. Zagreb, 2017. Faculty of Humanities and Social Sciences Hrvoje Gržina IDENTIFYING ORIGINALS OF ANALOG AND DIGITAL PHOTOGRAPHS DOCTORAL THESIS Supervisor: Ph. D. Hrvoje Stančić, Associate Professor Zagreb, 2017 O MENTORU Dr. sc. Hrvoje Stančić, izv. prof. rođen je 2. listopada 1970. u Zagrebu gdje je završio osnovnu i srednju školu. Diplomirao je 1996. godine studijske grupe Informatologija (smjer Opća informatologija) i Engleski jezik i književnost na Filozofskome fakultetu u Zagrebu. Godine 1996. prihvaćen je kao znanstveni novak na projektu koji se vodi na Odsjeku za informacijske znanosti Filozofskoga fakulteta u Zagrebu (broj znanstvenika 244003). Magistrirao je 2001. godine s temom Upravljanje znanjem i globalna informacijska infrastruktura. Doktorirao je 2006. s temom Teorijski model postojanog očuvanja autentičnosti elektroničkih informacijskih objekata. Od listopada 2011. u zvanju je izvanrednoga profesora, a od 2014. godine u zvanju znanstvenoga savjetnika. Predstojnik je Katedre za arhivistiku i dokumentalistiku od 2008. godine. Nastavu izvodi na preddiplomskom, diplomskom i doktorskom studiju informacijskih i komunikacijskih -

Name, a Novel
NAME, A NOVEL toadex hobogrammathon /ubu editions 2004 Name, A Novel Toadex Hobogrammathon Cover Ilustration: “Psycles”, Excerpts from The Bikeriders, Danny Lyon' book about the Chicago Outlaws motorcycle club. Printed in Aspen 4: The McLuhan Issue. Thefull text can be accessed in UbuWeb’s Aspen archive: ubu.com/aspen. /ubueditions ubu.com Series Editor: Brian Kim Stefans ©2004 /ubueditions NAME, A NOVEL toadex hobogrammathon /ubueditions 2004 name, a novel toadex hobogrammathon ade Foreskin stepped off the plank. The smell of turbid waters struck him, as though fro afar, and he thought of Spain, medallions, and cork. How long had it been, sussing reader, since J he had been in Spain with all those corkoid Spanish medallions, granted him by Generalissimo Hieronimo Susstro? Thirty, thirty-three years? Or maybe eighty-seven? Anyhow, as he slipped a whip clap down, he thought he might greet REVERSE BLOOD NUT 1, if only he could clear a wasp. And the plank was homely. After greeting a flock of fried antlers at the shevroad tuesday plied canticle massacre with a flash of blessed venom, he had been inter- viewed, but briefly, by the skinny wench of a woman. But now he was in Rio, fresh of a plank and trying to catch some asscheeks before heading on to Remorse. I first came in the twilight of the Soviet. Swigging some muck, and lampreys, like a bad dram in a Soviet plezhvadya dish, licking an anagram off my hands so the ——— woundn’t foust a stiff trinket up me. So that the Soviets would find out. -

On the Export Control of High Speed Imaging for Nuclear Weapons Applications
LA-UR-17-28408 Approved for public release; distribution is unlimited. Title: On The Export Control Of High Speed Imaging For Nuclear Weapons Applications Author(s): Watson, Scott Avery Altherr, Michael Robert Intended for: Report Issued: 2017-09-15 Disclaimer: Los Alamos National Laboratory, an affirmative action/equal opportunity employer, is operated by the Los Alamos National Security, LLC for the National Nuclear Security Administration of the U.S. Department of Energy under contract DE-AC52-06NA25396. By approving this article, the publisher recognizes that the U.S. Government retains nonexclusive, royalty-free license to publish or reproduce the published form of this contribution, or to allow others to do so, for U.S. Government purposes. Los Alamos National Laboratory requests that the publisher identify this article as work performed under the auspices of the U.S. Department of Energy. Los Alamos National Laboratory strongly supports academic freedom and a researcher's right to publish; as an institution, however, the Laboratory does not endorse the viewpoint of a publication or guarantee its technical correctness. On The Export Control of High-Speed Imaging for Nuclear Weapons Applications By Scott Watson, and Michael Altherr Los Alamos National Lab LA-UR-XYZQ 2017. Abstract Since the Manhattan Project, the use of high-speed photography, and its cousins flash radiography1 and schieleren photography have been a technological proliferation concern. Indeed, like the supercomputer, the development of high-speed photography as we now know it essentially grew out of the nuclear weapons program at Los Alamos2,3,4. Naturally, during the course of the last 75 years the technology associated with computers and cameras has been export controlled by the United States and others to prevent both proliferation among non-P5-nations and technological parity among potential adversaries among P5 nations. -

Hand-Held Schlieren Photography with Light Field Probes
Hand-held Schlieren Photography with Light Field probes The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Wetzstein, Gordon, Ramesh Raskar, and Wolfgang Heidrich. “Hand- held Schlieren Photography with Light Field probes.” In 2011 IEEE International Conference on Computational Photography (ICCP), 1-8. As Published http://dx.doi.org/10.1109/ICCPHOT.2011.5753123 Publisher Institute of Electrical and Electronics Engineers (IEEE) Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/79911 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike 3.0 Detailed Terms http://creativecommons.org/licenses/by-nc-sa/3.0/ Hand-Held Schlieren Photography with Light Field Probes Gordon Wetzstein1;2 Ramesh Raskar2 Wolfgang Heidrich1 1The University of British Columbia 2MIT Media Lab Abstract We introduce a new approach to capturing refraction in transparent media, which we call Light Field Back- ground Oriented Schlieren Photography (LFBOS). By op- tically coding the locations and directions of light rays emerging from a light field probe, we can capture changes of the refractive index field between the probe and a camera or an observer. Rather than using complicated and expen- sive optical setups as in traditional Schlieren photography we employ commodity hardware; our prototype consists of a camera and a lenslet array. By carefully encoding the color and intensity variations of a 4D probe instead of a diffuse 2D background, we avoid expensive computational process- ing of the captured data, which is necessary for Background Oriented Schlieren imaging (BOS). We analyze the bene- fits and limitations of our approach and discuss application scenarios. -
Imaging and Science Are Inextricably Connected, and Scientific Imaging Is As Old As Photography Itself
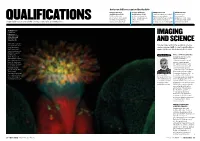
Get your skills recognised by the RPS Imaging Scientist Creative Industries REAP Distinction Film Distinction Qualifications (ISQ) Qualifications (CIQ) applicants submit an academic to support and for those who have for those working in the paper, essay or website, encourage the professional careers within media – including picture illustrated with images, in which production of innovative, the fields of engineering, editors, art directors they share knowledge and challenging, high-quality A special focus on one of the Society’s specialist accreditations science and technology and curators develop aspects of photography moving-image work ARABIDOPSIS Heiti Paves Department of Gene Technology, IMAGING Tallinn University of Technology, Tallinn, Estonia AND SCIENCE The flower of a thale cress plant revealed Crucial roles within the world of science using fluorescing are recognised with Society qualifications, dyes. The colours writes Professor Afzal Ansary ASIS FRPS come from chemicals that bind to specific proteins and AUTHOR PROFILE THE SOCIETY FACILITATES which fluoresce in learning and promotes the characteristic colours highest standards of when illuminated by a achievement in the art and laser. The thale cress science of photography (Arabidopsis thaliana) through its internationally is a useful model renowned Distinctions and organism in plant Qualifications programme. biology and genetics. Although science has always The beautiful been an integral part of the structure of the flower Society’s activities, in 1993 – in was captured using a Professor Afzal recognition of the lack of Zeiss confocal laser- Ansary ASIS FRPS is a vocational qualifications in the scanning microscope. Fenton Medallist and area of imaging science – it chair of the Society’s introduced four unique levels of imaging scientist Imaging Scientist Qualifications qualifications board (ISQ) for those professionally engaged in the field and its applications. -

Principles and Techniques of Schlieren Imaging Systems
PRINCIPLES AND TECHNIQUES OF SCHLIEREN IMAGING SYSTEMS Amrita Mazumdar Columbia University New York, NY [email protected] Originally released July 2011 June 18, 2013 Abstract This paper presents a review of modern-day schlieren optics system and its application. Schlieren imaging systems provide a powerful technique to visualize changes or nonuniformities in refractive index of air or other transparent media. For over two centuries, schlieren systems were typically implemented for a wide variety of non-intrusive real-time fluid dynamics studies. With the popularization of com- putational imaging techniques and widespread availability of digital imaging systems, schlieren systems provide novel methods of viewing transparent flow. Innovations such as background-oriented schlieren, rainbow schlieren interferometry, and synthetic schlieren imaging have built upon the fundamentals of schlieren imaging to provide less ambiguous, quantifiable studies of schlieren systems. This paper presents a historical background of the technique, describes the methodology behind the system, presents a mathematical proof of schlieren fundamentals, and lists various recent applications and advancements in schlieren studies. Contents 1 Introduction 2 2 History 3 3 Optical Theory 3 3.1 Basics Of Light Propagation In Schlieren Systems . 3 3.2 Refracting Light Through An Object . 4 1 4 Related Systems 5 4.1 Shadowgraphy . 5 4.2 Interferometry . 6 5 Experimental Implementation 7 5.1 Set-Up . 7 5.2 Captured Images . 8 5.3 Implementation and Verification . 9 5.4 Limitations . 10 6 Advanced Schlieren Techniques 11 6.1 Background-oriented Schlieren . 12 6.2 Resolution Improvements . 13 6.2.1 Spatial Resolution . 13 6.2.2 Dynamic Range . -

Computational Schlieren Photography with Light Field Probes
Computational Schlieren Photography with Light Field Probes The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Wetzstein, Gordon, Wolfgang Heidrich, and Ramesh Raskar. “Computational Schlieren Photography with Light Field Probes.” International Journal of Computer Vision 110, no. 2 (August 20, 2013): 113–127. As Published http://dx.doi.org/10.1007/s11263-013-0652-x Publisher Springer US Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/106832 Terms of Use Creative Commons Attribution-Noncommercial-Share Alike Detailed Terms http://creativecommons.org/licenses/by-nc-sa/4.0/ Int J Comput Vis (2014) 110:113–127 DOI 10.1007/s11263-013-0652-x Computational Schlieren Photography with Light Field Probes Gordon Wetzstein · Wolfgang Heidrich · Ramesh Raskar Received: 27 February 2013 / Accepted: 1 August 2013 / Published online: 20 August 2013 © Springer Science+Business Media New York 2013 Abstract We introduce a new approach to capturing refrac- der complex objects with synthetic backgrounds (Zongker et tion in transparent media, which we call light field back- al. 1999), or validate flow simulations with measured data. ground oriented Schlieren photography. By optically cod- Unfortunately, standard optical systems are not capable of ing the locations and directions of light rays emerging from recording the nonlinear trajectories that photons travel along a light field probe, we can capture changes of the refrac- in inhomogeneous media. In this paper, we present a new tive index field between the probe and a camera or an approach to revealing refractive phenomena by coding the observer. -

Lab 8: Shockwave Hydrodynamics by Laser Schlieren Photography
Lab 8: Shockwave Hydrodynamics by Laser Schlieren Photography Ian M. Rittersdorf NERS 575 Due 20 March 2008 Abstract The measured shockwave was found to have a velocity of 361 [m/s] and a Mach number of 1.0494. Using this value the ratio of pressures, temperatures, and densities of the air before and after the shockwave were measured. These values are 1.11816, 1.03247, and 1.08300, respectively. The shockwave was found to deposit 0.687 [mJ] of energy into the air. Contents 1 Introduction and Objectives 1 2 Experimental Configuration 1 3 Experimental Results 2 4 Data Analysis and Discussion 2 5 References 3 A Appendix 3 A.1 Analysis of Measurements . 3 A.2 Useful Equations . 3 A.3 Useful Measurements . 4 1 Introduction and Objectives Schlieren photography is a diagnostic process that can be used to measure the density of a gas. The purpose of this lab is to become familiar with Schlieren photography diagnostics and use the process to measure some parameters of air. 2 Experimental Configuration 1 4 DATA ANALYSIS AND DISCUSSION Figure 1: Schematic of setup. Figure 1 shows the basic setup of this experiment. A XeCl laser was fired through a plasma arc between two needles. A camera was set up to take pictures of the shockwave after various time delays between 0 and 10 [µs]. The knife edge is setup to eliminate all light from the photo except for diffracted light. 3 Experimental Results The raw data of this experiment was in the form of photographs, which were later analyzed. The photos themselves can be found at the following web address: http://www-personal.umich.edu/∼ianrit/ners575/ners575 thur 130.zip 4 Data Analysis and Discussion With the aide of NI Vision Assistant, the number of pixels of the diameter of the needles and the diameter of the shockwave was measured at each delay. -

Schlieren-Based Flow Imaging
Schlieren-Based Flow Imaging by Bradley Atcheson B.Sc., University of the Witwatersrand, 2004 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF Master of Science in The Faculty of Graduate Studies (Computer Science) The University Of British Columbia July, 2007 c Bradley Atcheson 2007 ii Abstract A transparent medium of inhomogenous refractive index will cause light rays to bend as they pass through it, resulting in a visible distortion of the background. We present a simple 2D imaging method for measuring this distortion and then show how it can be used to visualise gas and liquid flows. Improvements to the existing Background Oriented Schlieren method for acquiring projected density gradients are made by plac- ing a multi-scale wavelet noise pattern behind the flow and measuring distortions using a more reliable optical flow algorithm. Dynamic environment mattes can also be ac- quired, allowing us to render the flow into novel scenes. Capturing from multiple viewpoints then allows us to tomographically reconstruct 3D models of the tempera- ture distribution of the fluid. iii Contents Abstract ...................................... ii Contents ..................................... iii List of Tables ................................... v List of Figures .................................. vi Acknowledgements ............................... viii 1 Introduction ................................. 1 1.1 Motivation and Applications ...................... 3 1.2 Overview of the Method ........................ 6 1.3 Contribution ............................... 8 2 Schlieren Imaging .............................. 10 2.1 Optical Principle ............................ 10 2.1.1 Lens-Based Systems ...................... 11 2.2 Other Schlieren Configurations ..................... 14 2.2.1 Rainbow Schlieren ....................... 14 2.2.2 Grid-Based Systems ...................... 15 2.2.3 Background Oriented Schlieren ................ 16 2.3 BOS and Shadowgraphy ........................ 20 3 Optical Flow ................................