Lilly, Roche, Merck & Co, Astrazeneca, Wyeth, Bristol-Myers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Print Layout 1
TECHNICAL PROGRAM Monday, March 19 5:00 – 8:00 pm Welcome Reception Tuesday, March 20 8:00 – 10:00 Session 1: Setting the Conference Context and Drivers Chair: Geoff Slaff (Amgen) Roger Perlmutter (Amgen) Conquering the Innovation Deficit in Drug Discovery Helen Winkle (CDER, FDA) Regulatory Modernization 10:00 – 10:30 Break Vendor and poster review 10:30 – 12:30 Session 2: Rapid Cell Line Development and Improved Expression System Development Chairs: Timothy Charlebois (Wyeth) and John Joly (Genentech) Amy Shen (Genentech) Stable Antibody Production Cell-Line Development with an Improved Selection Process and Accelerated Timeline Mark Leonard (Wyeth) High-Performing Cell-Line Development within a Rapid and Integrated Platform Process Control Pranhitha Reddy (Amgen) Applying Quality-by-Design to Cell Line Development Lin Zhang (Pfizer) Development of a Fully-Integrated Automated System for High-Throughput Screening and Selection of Single Cells Expressing Monoclonal Antibodies 12:30 – 2:00 Lunch Vendor and poster review 2:00 – 4:30 Session 3: High-Throughput Bulk Process Development Chairs: Brian Kelley (Wyeth) and Jorg Thommes (Biogen Idec) Colette Ranucci (Merck) Development of a Multi-Well Plate System for High-Throughput Process Development Min Zhang (SAFC Biosciences) CHO Media Library – an Efficient Platform for Rapid Development and Optimization of Cell Culture Media Supporting High Production of Pharmaceutical Proteins in Chinese Hamster Ovary Cells Nigel Titchener-Hooker The Use of Ultra-Scale-Down Approaches to Enable Rapid Investigation -

Faculty Disclosure
Faculty Disclosure In accordance with the ACCME Standards for Commercial Support, course directors, planning committees, faculty and all others in control of the educational content of the CME activity must disclose all relevant financial relationships with any commercial interest that they or their spouse/partner may have had within the past 12 months. If an individual refuses to disclose relevant financial relationships, they will be disqualified from being a part of the planning and implementation of this CME activity. Owners and/or employees of a commercial interest with business lines or products relating to the content of the CME activity will not be permitted to participate in the planning or execution of any accredited activity. Nature of Relevant Financial Relationship Last Name Commercial Interest What Was Received For What Role AbbVie, Allergan/ Tobira Therapeutics Inc, Gilead Research Grant Research Balart Sciences Inc, Pfizer, Salix Pharmaceuticals AbbVie, Merck Honorarium Advisory Board Bau None N/A N/A Benz None N/A N/A AbbVie, Arbutus Biopharma, Dieterich Gilead Sciences, Inc., Bristol- Research Grant Consultant Myers Squibb, Merck Bayer HealthCare Pharmaceuticals, Gilead Sciences Honorarium Speaking, Consultant Inc. Bristol-Myers Squibb, Gilead Speaking, Advisory Sciences, Inc, Salix Honorarium Frenette Board Pharmaceuticals, Inc, Merck Intercept Pharmaceuticals Honorarium Advisor Conatus Pharmaceuticals Inc Honorarium Consulting Principle Investigator, Research Grant, Han Gilead Sciences, -

Ipo Virtual Annual Meeting Sept
PATENTS | TRADEMARKS | COPYRIGHTS TRADE SECRETS | INDUSTRIAL DESIGNS IPO VIRTUAL ANNUAL MEETING SEPT. 21-24, 2020 IPO.ORG/AM2020 KEYNOTE SPEAKERS 30+ EDUCATION POPPY CRUM SESSIONS PhD, Chief Scientist, Dolby Laboratories ANTÓNIO CAMPINOS President, European Patent Office IP EXPO #IPOAM20 ANDREI IANCU Under Secretary of Commerce for Intellectual Property and Director United States Patent and Trademark Office KASUTANI NETWORKING TOSHIHIDE Commissioner, Japan Patent Office CONNECT ENGAGE LEARN IPO VIRTUAL ANNUAL MEETING | SEPT. 21-24, 2020 TABLE OF CONTENTS ANNUAL MEETING STANDING IP COMMITTEES 4 COMMITTEE BUSINESS MEETINGS 5 PROGRAM AT-A-GLANCE 6 GENERAL SESSIONS 8 WORKSHOPS 11 PATENT SESSIONS 12 TRADEMARK/COPYRIGHT SESSIONS 17 INDUSTRIAL DESIGNS SESSIONS 20 OPERATIONS SESSIONS 21 SPONSORS 22 VIRTUAL IP EXPO 23 NETWORKING 24 REGISTRATION AND MEETING POLICIES 27 REGISTRATION FORM 28 IPO VIRTUAL ANNUAL MEETING | SEPT. 21-24, 2020 ANNUAL MEETING We are looking forward to bringing our members together in a new and innovative way this year for the 2020 Virtual Annual PROGRAM COMMITTEE Meeting on a secure and engaging virtual platform. The IPO Annual Meeting offers a mix of educational programs LEADERSHIP: featuring leaders in the IP industry, committee meetings, networking opportunities, exhibits, and more. This must-attend CHAIR: event brings together IP professionals from around the world Steve Caltrider, Eli Lilly and Co. to discuss strategies, trends, and best practices. More than VICE CHAIR FOR PATENTS: 30 education sessions will be offered on patents, trademarks, Scott Barker, Micron Technology, Inc. copyrights, industrial designs, and – new this year – operations. In addition to the sessions planned by IPO’s Program VICE CHAIR FOR TRADEMARKS: Committee, several sessions are being organized by IPO’s Colette Durst, 3M Innovative Properties Co. -

Bristol Myers
United States Court of Appeals for the Federal Circuit ______________________ BRISTOL-MYERS SQUIBB COMPANY, Plaintiff-Appellant, v. TEVA PHARMACEUTICALS USA, INC., Defendant-Appellee. ______________________ 2013-1306 ______________________ Appeal from the United States District Court for the District of Delaware in No. 10-CV-0805, Magistrate Judge Christopher J. Burke. ______________________ Decided: June 12, 2014 ______________________ WILLIAM F. LEE, Wilmer Cutler Pickering Hale and Dorr LLP, of Boston, Massachusetts, argued for plaintiff- appellant. With him on the brief were LAUREN B. FLETCHER and ANDREW J. DANFORD; AMY K. WIGMORE and THOMAS G. SAUNDERS, of Washington, DC. Of counsel on the brief were PAUL BERGHOFF, ALISON J. BALDWIN, and JOSHUA R. RICH, McDonnell Boehnen Hulbert & Berghoff LLP, of Chicago, Illinois. GEORGE C. LOMBARDI, Winston & Strawn LLP, of Chi- cago, Illinois, argued for defendant-appellee. With him on the brief were LYNN MACDONALD ULRICH, IVAN M. 2 BRISTOL-MYERS SQUIBB COMPANY v. TEVA PHARMACEUTICALS USA, INC. POULLAOS, JULIA MANO JOHNSON, and WILLIAM P. FERRANTI. ______________________ ∗ Before PROST, Chief Judge, PLAGER and CHEN, Circuit Judges. CHEN, Circuit Judge. This patent infringement case concerns a drug for the treatment of hepatitis B. After a four-day bench trial, the United States District Court for the District of Delaware found claim 8 of U.S. Patent No. 5,206,244 (’244 patent) invalid as obvious. We affirm the district court’s invalidi- ty judgment for the reasons that follow. I. Appellant Bristol-Myers Squibb Co. (BMS) owns the ’244 patent. Claim 8 of the ’244 patent is directed to a nucleoside analog composed of two regions: a carbocyclic ring and a guanine base. -

Investor Presentation March 19, 2019
Transaction Update INVESTOR PRESENTATION MARCH 19, 2019 1 Important Information For Investors And Stockholders This communication does not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote or approval. It does not constitute a prospectus or prospectus equivalent document. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the U.S. Securities Act of 1933, as amended. In connection with the proposed transaction between Bristol-Myers Squibb Company (“Bristol-Myers Squibb”) and Celgene Corporation (“Celgene”), on February 1, 2019, Bristol-Myers Squibb filed with the Securities and Exchange Commission (the “SEC”) a registration statement on Form S-4, as amended on February 1, 2019 and February 20, 2019, containing a joint proxy statement of Bristol-Myers Squibb and Celgene that also constitutes a prospectus of Bristol- Myers Squibb. The registration statement was declared effective by the SEC on February 22, 2019, and Bristol-Myers Squibb and Celgene commenced mailing the definitive joint proxy statement/prospectus to stockholders of Bristol-Myers Squibb and Celgene on or about February 22, 2019. INVESTORS AND SECURITY HOLDERS OF BRISTOL-MYERS SQUIBB AND CELGENE ARE URGED TO READ THE DEFINITIVE JOINT PROXY STATEMENT/PROSPECTUS AND OTHER DOCUMENTS FILED OR THAT WILL BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION. Investors and security holders will be able to obtain free copies of the registration statement and the definitive joint proxy statement/prospectus and other documents filed with the SEC by Bristol-Myers Squibb or Celgene through the website maintained by the SEC at http://www.sec.gov. -

FDA Listing of Authorized Generics As of July 1, 2021
FDA Listing of Authorized Generics as of July 1, 2021 Note: This list of authorized generic drugs (AGs) was created from a manual review of FDA’s database of annual reports submitted to the FDA since January 1, 1999 by sponsors of new drug applications (NDAs). Because the annual reports seldom indicate the date an AG initially entered the market, the column headed “Date Authorized Generic Entered Market” reflects the period covered by the annual report in which the AG was first reported. Subsequent marketing dates by the same firm or other firms are not included in this listing. As noted, in many cases FDA does not have information on whether the AG is still marketed and, if not still marketed, the date marketing ceased. Although attempts have been made to ensure that this list is as accurate as possible, given the volume of d ata reviewed and the possibility of database limitations or errors, users of this list are cautioned to independently verify the information on the list. We welcome comments on and suggested changes (e.g., additions and deletions) to the list, but the list may include only information that is included in an annual report. Please send suggested changes to the list, along with any supporting documentation to: [email protected] A B C D E F G H I J K L M N O P Q R S T U V X Y Z NDA Applicant Date Authorized Generic Proprietary Name Dosage Form Strength Name Entered the Market 1 ACANYA Gel 1.2% / 2.5% Bausch Health 07/2018 Americas, Inc. -

Bristol-Myers Squibb/Pfizer Alliance Independent Medical Education/Global Medical Grants Request for Educational Support (RFE)
Bristol-Myers Squibb/Pfizer Alliance Independent Medical Education/Global Medical Grants Request for Educational Support (RFE) Date September 4, 2019 RFE Requestor Information Name: Sylvia Nashed, PharmD, RPh Title: IME Specialist Phone: 609-302-3320 E-mail: [email protected] RFE Code RFE-19-CV-104 Therapeutic Area Cardiovascular Area of Interest Treatment of Venous Thromboembolism (VTE) in Cancer It is our intent to support educational initiatives that focus on educating practitioners on optimal management of patients with VTE who also have cancer (treatment and reduction in risk of recurrence following initial treatment). This grant will be awarded for creation of VTE in Cancer learnings and tools to be used as part of a comprehensive initiative to address the educational gaps among HCPs in providing care to VTE patients with cancer. The successful proposal will have: Clear and concise statement of the goal, learning objectives, and expected outcomes of the educational initiative Learning plan that incorporates innovative techniques designed to engage learners, promotes application of education into practice, and incorporates the patient voice into educational resources Tools that provide HCP learners the opportunity to facilitate change to improve patient outcomes Measurement of outcomes, inclusive of learner progression throughout the activity, extent to which the activity closed the identified practice gaps, and patient impact Educational Design Bristol-Myers Squibb/Pfizer Alliance is interested in supporting a comprehensive educational initiative that is innovative, engaging, interactive, and that leverages current scientific evidence to improve HCP knowledge, skills, competence, and ultimately patient care. The activity(ies) should measure improvement of learners’ knowledge, confidence, competence, and performance and should achieve at least a Moore’s Level 4 impact. -
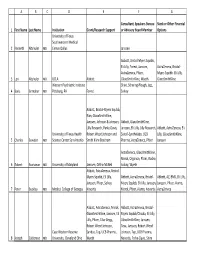
1 2 3 4 5 6 7 8 ABCDEFG First Name Last Name
ABC D E F G Consultant, Speakers Bureau Stock or Other Financial 1 First Name Last Name Institution Grant/Research Support or Advisory Board Member Options University of Texas Southwestern Medical 2 Kenneth Altshuler MD Center Dallas Janssen Abbott, Bristol‐Myers Squibb, Eli Lilly, Forest, Janssen, AstraZeneca, Bristol‐ AstraZeneca, Pfizer, Myers Squibb. Eli Lilly, 3 Lori Altshuler MD UCLA Abbott GlaxoSmithKline, Wyeth GlaxoSmithKline Western Psychiatric Institute Shire, Schering‐Plough, Jazz, 4 Boris Birmaher MD Pittsburg, PA Forest Solvay Abbott, Bristol‐Myers Squibb, Elan, GlaxoSmithKline, Janssen, Johnson & Johnson, Abbott, GlaxoSmithKline, Lilly Research, Parke‐Davis, Janssen, Eli Lilly, Lilly Research, Abbott, AstraZeneca, Eli University of Texas Health Robert Wood Johnson and Sanofi‐Synthelabo, UCB Lilly, GlaxoSmithKline, 5 Charles Bowden MD Science Center San Antonio Smith Kline Beecham Pharma, AstraZeneca, Pfizer Janssen AstraZeneca, GlaxoSmithKline, Merck, Organon, Pfizer, Roche, 6 Robert Buchanan MD University of Maryland Janssen, Ortho‐McNeil Solvay, Wyeth Abbott, AstraZeneca, Bristol‐ Myers Squibb, Eli Lilly, Abbott, AstraZeneca, Bristol‐ Abbott, AZ, BMS, Eli Lilly, Janssen, Pfizer, Solvay, Myers Squibb, Eli Lilly, Janssen, Janssen, Pfizer, Alamo, 7 Peter Buckley MD Medical College of Georgia Novartis Merck, Pfizer, Alamo, Novartis AstraZeneca Abbott, AstraZeneca, Merck, Abbott, AstraZeneca, Bristol‐ GlaxoSmithKline, Janssen, Eli Myers Squibb/Otsuka, Eli Lilly, Lilly, Pfizer, Ciba‐Geigy, GlaxoSmithKline, Janssen, Robert Wood -

Op0227 Efficacy of Deucravacitinib, an Oral, Selective Tyrosine Kinase 2 Inhibitor, in Musculoskeletal Manifestations of Active Psoriatic Arthritis
Ann Rheum Dis: first published as 10.1136/annrheumdis-2021-eular.2653 on 19 May 2021. Downloaded from Scientific Abstracts 137 Disclosure of Interests: Maria-Antonietta D’Agostino Speakers bureau: Sanofi, deucravacitinib was efficacious and well tolerated versus placebo (PBO) in Novartis, BMS, Janssen, Celgene, AbbVie, UCB pharma and Eli Lilly, Consultant patients with active psoriatic arthritis (PsA). of: Sanofi, Novartis, BMS, Janssen, Celgene, AbbVie, UCB pharma and Eli Lilly, Objectives: This analysis further evaluated improvements in musculoskeletal Philip G Conaghan Speakers bureau: AbbVie, AstraZeneca, BMS, Eli Lilly, Gal- disease manifestations in patients in the Phase 2 PsA trial. apagos, Gilead, Novartis and Pfizer, Consultant of: AbbVie, AstraZeneca, BMS, Methods: The ongoing Phase 2 trial (NCT03881059) enrolled patients who had Eli Lilly, Galapagos, Gilead, Novartis and Pfizer, Corine Gaillez Shareholder of: a PsA diagnosis for ≥6 months, met CASPAR criteria, had active disease (≥3 ten- Novartis and BMS, Employee of: Novartis, Maarten Boers Consultant of: BMS, der joints, ≥3 swollen joints, C-reactive protein [CRP] ≥3 mg/L), and had at least 1 Novartis, Pfizer, and GSK, Esperanza Naredo Speakers bureau: AbbVie, Roche, active skin lesion. Patients either failed or were intolerant to at least 1 nonsteroi- BMS, Pfizer, UCB, Eli Lilly, Novartis, Janssen and Celgene, Consultant of: Abb- dal anti-inflammatory drug, corticosteroid, conventional synthetic disease-mod- Vie, Novartis and BMS, Grant/research support from: Eli Lilly, Philippe Carron ifying antirheumatic drug, and/or 1 TNF inhibitor (TNFi; ≤30%). Patients were Speakers bureau: Pfizer, MSD, Novartis, BMS, AbbVie, UCB, Eli Lilly, Gilead randomized 1:1:1 to deucravacitinib 6 mg QD or 12 mg QD or PBO, and stratified and Celgene, Consultant of: Pfizer, MSD, Novartis, BMS, AbbVie, UCB, Eli Lilly, by TNFi status (experienced vs naive) and body weight (<90 vs ≥90 kg). -

Johnson & Johnson Qui Tam Relators Complaint
UNITED STATES DISTRICT COURT DISTRICT OF MASSACHUSETTS ZOUl NOV - I A II: lb' UNITED STATES OF AMERICA ex rei. ) BERNARD LISITZA, STATE OF ILLINOIS ex ) rei. BERNARD LISITZA, STATE OF ) CALIFORNIA ex rei. BERNARD LISITZA, ) STATE OF DELAWARE ex rei. BERi\fARD ) LISITZA, DISTRICT OF COLUMBIA ex rei. ) BERNARD LISITZA, STATE OF FLORIDA ex ) No.07-10288-RGS rei. BERNARD LISITZA, STATE OF GEORGIA) ex rei. BERNARD LISITZA, STATE OF HAWAll ) ex rel. BERNARD LISITZA, STATE OF ) INDIANA ex rei. BERNARD LISITZA, STATE ) FILED UNDER SEAL OF LOUISIANA ex rei. BERNARD LISITZA, ) COMMONWEALTH OF MASSACHUSETTS ex ) JURY TRIAL DEMANDED rei. BERNARD LISITZA, STATE OF MICHIGAN) ex rei. BERNARD LISITZA, STATE OF ) NEVADA ex rei. BERNARD LISITZA, STATE ) OF NEW HAMPSHIRE ex rei. BERNARD ) LISITZA, STATE OF NEW MEXICO ex rei. ) BERNARD LISITZA, STATE OF NEW YORK ex ) rei. BERNARD LISITZA, STATE OF ) TENNESSEE ex rei. BERNARD LISITZA, ) STATE OF TEXAS ex rei. BERNARD LISITZA, ) COMMONWEALTH OF VIRGINIA ex rei. ) BERNARD LISITZA, and BERNARD LISITZA, ) individually, ) ) Plaintiffs, ) ) v. ) ) PFIZER, INC., BRISTOL MYERS SQUIBB, CO., ) JOHNSON & JOHNSON, ORTHO-MCNEIL ) PHARMACEUTICALS, INC., and JANSSEN, LP, ) Defendants. SECOND AMENDED COMPLAINT I. INTRODUCTION ................................................................................................................. 1 n. PARTIES .............................................................................................. ,', .. , ............... , .. ,"" 6 III. JURISDICTION AND VENUE ..... , ............................... -

[email protected] [email protected] MARK A
Case 5:19-cv-02573-SVK Document 1 Filed 05/14/19 Page 1 of 136 1 DURIE TANGRI LLP HILLIARD & SHADOWEN LLP DARALYN J. DURIE (SBN 169825) STEVE D. SHADOWEN (pro hac vice pending) 2 [email protected] [email protected] MARK A. LEMLEY (SBN 155830) ROBERT C. HILLIARD (pro hac vice pending) 3 [email protected] [email protected] DAVID McGOWAN (SBN 154289) RICHARD BRUNELL (pro hac vice pending) 4 [email protected] [email protected] LAURA E. MILLER (SBN 271713) MATTHEW C. WEINER (pro hac vice pending) 5 [email protected] [email protected] W. HENRY HUTTINGER (SBN 312843) FRAZAR W. THOMAS (pro hac vice pending) 6 [email protected] [email protected] 217 Leidesdorff Street NICHOLAS W. SHADOWEN (pro hac vice pending) 7 San Francisco, CA 94111 [email protected] Telephone: (415) 362-6666 1135 W. 6th Street, Suite 125 8 Facsimile: (415) 236-6300 Austin, TX 78703 Telephone: (855) 344-3298 9 (see additional counsel on next page) Facsimile: (361) 882-3015 10 Attorneys for Plaintiffs Peter Staley, Steve Fuller, Gregg S. Gonsalves, PhD, Brenda Emily Goodrow, Andrew R. Spieldenner, PhD, Robert J. Vazquez, 11 Jason Walker, Michael Warner, Jacob Zydonis, Fraternal Order of Police, Fort Lauderdale Lodge 31, Insurance Trust Fund, and Service Employees 12 International Union, Local No. 1 Health Fund 13 IN THE UNITED STATES DISTRICT COURT 14 FOR THE NORTHERN DISTRICT OF CALIFORNIA 15 PETER STALEY; STEVE FULLER; GREGG S. GONSALVES, PhD; BRENDA EMILY GOODROW; 16 ANDREW R. SPIELDENNER, PhD; ROBERT J. VAZQUEZ; JASON WALKER; MICHAEL 17 WARNER; JACOB ZYDONIS; FRATERNAL ORDER OF POLICE, FORT LAUDERDALE 18 LODGE 31, INSURANCE TRUST FUND; and SERVICE EMPLOYEES INTERNATIONAL Case No. -

Sign-Up Form for the Bristol-Myers Squibb Patient Assistance Foundation
Sign-up Form for the Bristol-Myers Squibb Patient Assistance Foundation What is the Bristol-Myers Squibb Patient Assistance Foundation? • Bristol-Myers Squibb Company (BMS) established the Bristol-Myers Squibb Patient Assistance Foundation, Inc. (BMSPAF) to help patients who need help paying for medicines prescribed by their healthcare providers. BMSPAF is a non-profit organization that helps certain patients get, free of charge, the medicines that are listed in this application. • Patients who meet certain rules will be able to get their prescribed medicines free of charge for up to one year. Every year, you must reapply, and be accepted, to continue in the program. • Once approved, most medicine will be shipped to your home or to your healthcare provider’s office. What medications are available from the Foundation? ABILIFY® (aripiprazole) NULOJIX® (belatacept) BYDUREON® (exenatide extended-release for injectable suspension) ONGLYZA® (saxagliptin) BYETTA® (exenatide) ORENCIA® (abatacept) ELIQUIS® (apixaban) SymlinPen® (pramlintide acetate) Pen Injector TM KOMBIGLYZE XR (saxagliptin and metformin HCl extended-release) Am I able to get medication free of charge? You may be able to get medicine free of charge through the Bristol-Myers Squibb Patient Assistance Foundation if: • You are being treated as an outpatient with one of the medicines listed in this application. • You live in the USA, Puerto Rico, or the U.S. Virgin Islands. • You meet the income limits for your medicine. You will need to send your most recent Federal Tax Return or other proof of income. • You do not have insurance coverage for your BMS medicines or you are signed up for a Medicare Part D plan and have spent at least 3% of your yearly household income on out-of-pocket costs for prescription medicines this year.