Millikan's Oil Drop Experiment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Millikan's Struggle with Theory
FEATURES Did the great experimentalist doctor his own findings to suit his beliefs? Perhaps not, as aclose look at his beliefs reveals. Millikan's struggle with theory Gerald Holton n 1916 Millikan published his experi by any other experimenter. rectly at the ordinates, he decided"simply I mental determination of the value of Several factors were responsible for the to cut the feet off"where those curves were Planck's constant, with remarkable preci success of the experiment, performed at trailing on too far. sion. Itwas later cited as part ofhis Nobel the Ryerson Laboratory ofthe University In short, a triumphant work by a su Prize award. But as a study of that paper ofChicago. First ofall, Millikan's use ofan perbly confident scientist, ofhighest im together with Millikan's subsequent com ingenious device he termed "a machine portance in its day, and richly deserving to ments over the years reveal, he initiallyre shop in vacuo;' assembled thanks to "the be cited as part of his Nobel Prize award fused to accept the interpretation of the skill and experience of the mechanician, in 1923, "for his work on the elementary theoretical meaning ofhis work, as seen Mr. Julius Pearson" .Essentially, the device charge ofelectricityandthe photoelectric from the perspective of a present-day allowed a rotating sharp knife, controlled effect". If historically minded scholars physicist.Yet, over time, Millikan adjusted from outside the evacuated glass contain look through that 1916 volume of the his view retroactively to accept the mean er by electromagnetic means, to clean off Physical Review inwhich Millikan's paper ing ofwhat he had done - namely to pro the surface of the metal used (sodium, appeared, they will notice that physics in vide crucial support for Einstein's heuris potassium, lithium) before exposing it to America at that time was still a mixed bag. -

Millikan Oil Drop Experiment
Experiment 4 1 Millikan Oil-Drop Experiment Physics 2150 Experiment 4 University of Colorado1 Introduction The fundamental unit of charge is the charge of an electron, which has the magnitude of 푒 = 1.60219 x 10-19 C. With the exception of quarks, all elementary particles observed in nature are found to have a charge equal to an integral multiple of the charge of an electron. (Quarks have charge 푒/3, but they are never observed as free particles). By convention, the symbol 푒 represents a positive charge, so the charge 푞 of an electron is 푞 = −푒. The first person to measure accurately the electron’s charge was the American physicist R. A. Millikan. Working at the University of Chicago in 1912, Millikan found that droplets of oil from a simple spray bottle usually carry a net charge of a few electrons. He built an apparatus in which tiny oil droplets fall between two horizontal capacitor plates while their motion is observed with a microscope. A voltage applied between the plates creates an electric field, which can exert an upward force on a charge droplet, counteracting the force of gravity. By studying the motion of a droplet in response to gravity, the electric field between the plates, and viscous drag due to the air, Millikan was able to calculate the charge on the droplet. He published data on 58 droplets and reported that the charge of an electron is 푒 = (1.592±0.003) x 10-19 C. Millikan’s value was a bit low (three 휎 below the accepted value) because he used a slightly inaccurate expression for the drag force due to air. -

Tournament-9 Round 12 Tossups 1
Tournament-9 Round 12 Tossups 1. This psychologist argued that "moving toward others," "moving against others," and "moving away from others" were the three unhealthy trends. She organized the Association for the Advancement of Psychoanalysis after being expelled from the New York Psychoanalytic Institute. She argued against the concept of the death instinct and libido in works such as Feminine Psychology and The Neurotic Personality of Our Time. (*) For 10 points, name this psychologist, who introduced the concept of womb envy. ANSWER: Karen Horney 030-09-6-12102 2. The magnitudes of constructs of this type are related to J-coupling constants in proton NMR spectroscopy, according to the Karplus equations. One construct of this type is also known as the "dihedral" type, and applies when four or more atoms are bonded in a straight chain. For a central molecule with two sigma bonds, the magnitude of a construct of this type (*) decreases by one-third when a lone pair is present, which is the major difference between a linear and a bent molecule. For 10 points, name these figures important in molecular geometry, which come in "bond" and "torsional" varieties, and are typically measured in degrees. ANSWER: angles [or torsional angles; or bond angles; or dihedral angles] 026-09-6-12103 3. This deity trips a figure named Lit and kicks him into a pyre. This deity, assisted by some items given by Grid, defeats Geirrod, but fails to wrestle Eli, who is really Old Age. This deity cannot pick up the cat of (*) Utgard-Loki, but does make Thialfi into his apprentice. -

Moremore Millikan’S Oil-Drop Experiment
MoreMore Millikan’s Oil-Drop Experiment Millikan’s measurement of the charge on the electron is one of the few truly crucial experiments in physics and, at the same time, one whose simple directness serves as a standard against which to compare others. Figure 3-4 shows a sketch of Millikan’s apparatus. With no electric field, the downward force on an oil drop is mg and the upward force is bv. The equation of motion is dv mg Ϫ bv ϭ m 3-10 dt where b is given by Stokes’ law: b ϭ 6a 3-11 and where is the coefficient of viscosity of the fluid (air) and a is the radius of the drop. The terminal velocity of the falling drop vf is Atomizer (+) (–) Light source (–) (+) Telescope Fig. 3-4 Schematic diagram of the Millikan oil-drop apparatus. The drops are sprayed from the atomizer and pick up a static charge, a few falling through the hole in the top plate. Their fall due to gravity and their rise due to the electric field between the capacitor plates can be observed with the telescope. From measurements of the rise and fall times, the electric charge on a drop can be calculated. The charge on a drop could be changed by exposure to x rays from a source (not shown) mounted opposite the light source. (Continued) 12 More mg Buoyant force bv v ϭ 3-12 f b (see Figure 3-5). When an electric field Ᏹ is applied, the upward motion of a charge Droplet e qn is given by dv Weight mg q Ᏹ Ϫ mg Ϫ bv ϭ m n dt Fig. -

Millikan Oil Drop Experiment
Agenda 1. Measuring of the charge of electron. 2. Robert Millikan and his oil drop experiment 3. Theory of the experiment 4. Laboratory setup 5. Data analysis 2/17/2014 2 Measuring of the charge of the electron 1. Oil drop experiment. Robert A. Millikan.. (1909). e=1.5924(17)×10−19 C 2. Shot noise experiment. First proposed by Walter H. Schottky 3. In terms of the Avogadro constant and Faraday constant 풆 = 푭 ; F- Faraday constant, NA - Avagadro constant. Best 푵푨 uncertainty ~1.6 ppm. ퟐ풆 풉 4. From Josephson (푲 = ) and von Klitzing 푹 = constants 푱 풉 푲 풆ퟐ 5. Recommended by NIST value 1.602 176 565(35) 10-19 C 2/17/2014 3 Robert Millikan. Oil drop experiment The Nobel Prize in Physics 1923. Robert A. Millikan "for his work on the elementary charge of electricity and on the photoelectric effect". ROBERT ANDREWS MILLIKAN 1868-1953 22nd of March, 1868, Morrison, Ill University of Chicago 2/17/2014 4 Robert Millikan. Oil drop experiment ROBERT ANDREWS MILLIKAN 1868-1953 Diagram and picture of apparatus 2/17/2014 5 Oil drop experiment. Measurement of the magnitude of the electron charge! Motivation: Demonstrate that the electron charge is quantized! Measure the charge of an electron to ±3% Picture of the PASCO setup 2/17/2014 6 Oil drop experiment. atomizer Oil drops ∅~1m telescope + V Vg d 500V - 흆풂풊풓 Forces on the oil drop: 1) Gravity + buoyant force (air displaced by oil drop) 2) Drag force of the oil drop in the air 2/17/2014 7 Oil drop experiment. -

Settling Velocities of Particulate Systems†
Settling Velocities of Particulate Systems† F. Concha Department of Metallurgical Engineering University of Concepción.1 Abstract This paper presents a review of the process of sedimentation of individual particles and suspensions of particles. Using the solutions of the Navier-Stokes equation with boundary layer approximation, explicit functions for the drag coefficient and settling velocities of spheres, isometric particles and arbitrary particles are developed. Keywords: sedimentation, particulate systems, fluid dynamics Discrete sedimentation has been successful to 1. Introduction establish constitutive equations in processes using Sedimentation is the settling of a particle, or sus- sedimentation. It establishes the sedimentation prop- pension of particles, in a fluid due to the effect of an erties of a certain particulate material in a given fluid. external force such as gravity, centrifugal force or Nevertheless, to analyze a sedimentation process and any other body force. For many years, workers in to obtain behavioral pattern permitting the prediction the field of Particle Technology have been looking of capacities and equipment design procedures, an- for a simple equation relating the settling velocity of other approach is required, the so-called continuum particles to their size, shape and concentration. Such approach. a simple objective has required a formidable effort The physics underlying sedimentation, that is, the and it has been solved only in part through the work settling of a particle in a fluid is known since a long of Newton (1687) and Stokes (1844) of flow around time. Stokes presented the equation describing the a particle, and the more recent research of Lapple sedimentation of a sphere in 1851 and that can be (1940), Heywood (1962), Brenner (1964), Batchelor considered as the starting point of all discussions of (1967), Zenz (1966), Barnea and Mitzrahi (1973) the sedimentation process. -

Gravity and Projectile Motion
Do Now: What is gravity? Gravity Gravity is a force that acts between any 2 masses. Two factors affect the gravitational attraction between objects: mass and distance. What direction is Gravity? Earth’s gravity acts downward toward the center of the Earth. What is the difference between Mass and Weight? • Mass is a measure of the amount of matter (stuff) in an object. • Weight is a measure of the force of gravity on an object. • Your mass never changes,no matter where you are in the universe. But your weight will vary depending upon the force of gravity pulling down on you. Would you be heavier or lighter on the Moon? Gravity is a Law of Science • Newton realized that gravity acts everywhere in the universe, not just on Earth. • It is the force that makes an apple fall to the ground. • It is the force that keeps the moon orbiting around Earth. • It is the force that keeps all the planets in our solar system orbiting around the sun. What Newton realized is now called the Law of Universal Gravitation • The law of universal gravitation states that the force of gravity acts between all objects in the universe. • This means that any two objects in the universe, without exception, attract each other. • You are attracted not only to Earth but also to all the other objects around you. A cool thing about gravity is that it also acts through objects. Free Fall • When the only force acting on an object is gravity, the object is said to be in free fall. -

Millikan Oil Drop Experiment
(revised 1/23/02) MILLIKAN OIL-DROP EXPERIMENT Advanced Laboratory, Physics 407 University of Wisconsin Madison, Wisconsin 53706 Abstract The charge of the electron is measured using the classic technique of Millikan. Mea- surements are made of the rise and fall times of oil drops illuminated by light from a Helium{Neon laser. A radioactive source is used to enhance the probability that a given drop will change its charge during observation. The emphasis of the experiment is to make an accurate measurement with a full analysis of statistical and systematic errors. Introduction Robert A. Millikan performed a set of experiments which gave two important results: (1) Electric charge is quantized. All electric charges are integral multiples of a unique elementary charge e. (2) The elementary charge was measured and found to have the value e = 1:60 19 × 10− Coulombs. Of these two results, the first is the most significant since it makes an absolute assertion about the nature of matter. We now recognize e as the elementary charge carried by the electron and other elementary particles. More precise measurements have given the value 19 e = (1:60217733 0:00000049) 10− Coulombs ± × The electric charge carried by a particle may be calculated by measuring the force experienced by the particle in an electric field of known strength. Although it is relatively easy to produce a known electric field, the force exerted by such a field on a particle carrying only one or several excess electrons is very small. For example, 14 a field of 1000 volts per cm would exert a force of only 1:6 10− N on a particle × 12 bearing one excess electron. -
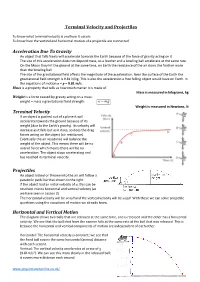
Terminal Velocity and Projectiles Acceleration Due to Gravity
Terminal Velocity and Projectiles To know what terminal velocity is and how it occurs To know how the vertical and horizontal motion of a projectile are connected Acceleration Due To Gravity An object that falls freely will accelerate towards the Earth because of the force of gravity acting on it. The size of this acceleration does not depend mass, so a feather and a bowling ball accelerate at the same rate. On the Moon they hit the ground at the same time, on Earth the resistance of the air slows the feather more than the bowling ball. The size of the gravitational field affects the magnitude of the acceleration. Near the surface of the Earth the gravitational field strength is 9.81 N/kg. This is also the acceleration a free falling object would have on Earth. In the equations of motion a = g = 9.81 m/s. Mass is a property that tells us how much matter it is made of. Mass is measured in kilograms, kg Weight is a force caused by gravity acting on a mass: weight = mass x gravitational field strength w mg Weight is measured in Newtons, N Terminal Velocity If an object is pushed out of a plane it will accelerate towards the ground because of its weight (due to the Earth’s gravity). Its velocity will increase as it falls but as it does, so does the drag forces acting on the object (air resistance). Eventually the air resistance will balance the weight of the object. This means there will be no overall force which means there will be no acceleration. -

Eastern Kentucky University Department of Physics and Astronomy Physics 406W TBD Advanced Physics Laboratory Writing Intensive CRN: TBD 3 Credit Hours
Eastern Kentucky University Department of Physics and Astronomy Physics 406W TBD Advanced Physics Laboratory Writing Intensive CRN: TBD 3 Credit Hours Course Time and Location: NSB3165 Instructor: Dr. Marco Ciocca Office: NSB 3162 Phone: 622-6172 Email: [email protected] Office Hours: MWF 10-11 AM or any other times in which I am in my office. Feel free to stop by! Catalog Description: PHY 406W Advanced Physics Laboratory. (3) A. Prerequisites: ENG 102, 105(B), or HON 102; PHY 132, 202, or departmental approval. Experiments in mechanics, optics, electricity, and nuclear physics. Computer simulations of physical situations. Measurement and data analysis techniques, including error analysis. Credit will not be awarded to students who have credit for PHY 406. 5 Lec/Lab. Experiments will be performed with the basic laboratory goals of a) How measurements are made. b) How data is analyzed, including how data is graphed, and c) How to draw quantitative conclusions from data in tabular and graphical form. d) Indicate the reliability and confidence in the data acquired through an appropriate error analysis. e) Demonstrate a good grasp of a-d by communicating effectively in writing. Last Date to withdraw from full semester classes: as per University policy. Textbook: “An Introduction to Error Analysis” 2nd edition by John R. Taylor. For the experiments we’ll use handouts. You must have a 3-ring binder or folder to keep you handout neatly. You will need a flash drive to keep your results. You will need loose-leaf sheet for your 3-ring binder so your sketches, table and any information you jot down during an experiment can be recorded. -

CALTECH NEWS PUB LIS H E D for a L U M N I a N D F R Len D S 0 F the CAL I FO R N I a INS TIT UTE of Tee H N 0 LOG Y
VOLUME 9, NUMBER B, NOVEMBER 1975 CALTECH NEWS PUB LIS H E D FOR A L U M N I A N D F R lEN D S 0 F THE CAL I FO R N I A INS TIT UTE OF TEe H N 0 LOG Y First Kenan Judge Shirley Hufstedler Professor: named to Caltech Board Harry B. Gray The nation's highest ranking as in education, Judge Hufstedler has woman judge, U. S. Circuit Judge often affirmed her belief that there is Dr. Harry B. Gray, 39, Caltech Shirley Hufstedler of the Ninth Cir nothing in the daily lives of individu chemistry professor for the past ten cuit Court of Appeals, has been als that is not touched by the law and years, has been named the first Wil elected to the CaItech Board of Trust the processes of justice. liam R. Kenan, Jr. Professor. ees, according to an announcement Describing herself as inde "The selection of Harry Gray as the by R. Stanton Avery, chairman. pendent-minded, she has said, "I've first occupant of this chair is a tribute The second woman in U. S. history participated in the women's rights re to both his charismatic qualities as a to reach that level in the judiciary, naissance all my life. I have always teacher and to his leadership in scien Judge Hufstedler has served in her believed that all human beings, in tific research," said President Harold present position since 1968. Prior to cluding women, should have oppor Brown in announcing the appoint that, she was a justice of California's tunities to make the best of their ment. -

Terminal Velocity
Terminal Velocity D. Crowley, 2008 2018年10月23日 Terminal Velocity ◼ To understand terminal velocity Terminal Velocity ◼ What are the forces on a skydiver? How do these forces change (think about when they first jump out; during free fall; and when the parachute has opened)? ◼ What happens if the skydiver changes their position? ◼ The skydiver’s force (Fweight=mg) remains the same throughout the jump ◼ But their air resistance changes depending upon what they’re doing which changes the overall resultant force Two Most Common Factors that Affect Air Resistance ◼Speed of the Object ◼Surface Area Air Resistance ◼ More massive objects fall faster than less massive objects Since the 150-kg skydiver weighs more (experiences a greater force of gravity), it will accelerate to higher speeds before reaching a terminal velocity. Thus, more massive objects fall faster than less massive objects because they are acted upon by a larger force of gravity; for this reason, they accelerate to higher speeds until the air resistance force equals the gravity force. Skydiving ◼ Falling objects are subject to the force of gravity pulling them down – this can be calculated by W=mg Weight (N) = mass (kg) x gravity (N/kg) ◼ On Earth the strength of gravity = 9.8N/kg ◼ On the Moon the strength of gravity is just 1.6N/kg Positional ◼ What happens when you change position during free-fall? ◼ Changing position whilst skydiving causes massive changes in air resistance, dramatically affecting how fast you fall… Skydiving Stages ◼ Draw the skydiving stages ◼ Label the forces