Effects of Prolonged Pneumoperitoneum on Hepatic Perfusion During Laparoscopy Lisette T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Massive Pneumoperitoneum Presenting As an Incidental Finding
Open Access Case Report DOI: 10.7759/cureus.2787 Massive Pneumoperitoneum Presenting as an Incidental Finding Harry Wang 1 , Vivek Batra 2 1. Internal Medicine, Thomas Jefferson University Hospitals, Philadelphia, USA 2. Medical Oncology, Thomas Jefferson University Hospital, Philadelphia, USA Corresponding author: Harry Wang, [email protected] Abstract Pneumoperitoneum is often associated with surgical complications or intra-abdominal sepsis. While commonly deemed a surgical emergency, pneumoperitoneum in a minority of cases does not involve a viscus perforation or require urgent surgical management; these cases of “spontaneous pneumoperitoneum” can stem from a variety of etiologies. We report a case of a 72-year-old African American male with a history of metastatic pancreatic adenocarcinoma who presented with new-onset abdominal distention and an incidentally discovered massive pneumoperitoneum with no clear source of perforation on surveillance imaging. His exam was non-peritonitic, so no surgical intervention was recommended. He was treated with bowel rest, intravenous antibiotics, and hydration. He had a relatively benign clinical course with preserved gastrointestinal function and had complete resolution of his pneumoperitoneum on imaging two months after discharge. This case highlights the importance of considering non-surgical causes of pneumoperitoneum, as well as conservative management, when approaching patients with otherwise benign abdominal exams. Categories: Internal Medicine, Gastroenterology, Oncology Keywords: spontaneous pneumoperitoneum, pneumoperitoneum, pancreatic cancer, incidental finding Introduction Pneumoperitoneum is defined as the presence of free air within the peritoneal cavity. In the vast majority of cases (approximately 90%), this is a result of an intra-abdominal viscus perforation, often requiring intravenous antibiotics and acute surgical intervention [1]. -

Spontaneous Pneumoperitoneum with Duodenal
Ueda et al. Surgical Case Reports (2020) 6:3 https://doi.org/10.1186/s40792-019-0769-4 CASE REPORT Open Access Spontaneous pneumoperitoneum with duodenal diverticulosis in an elderly patient: a case report Takeshi Ueda* , Tetsuya Tanaka, Takashi Yokoyama, Tomomi Sadamitsu, Suzuka Harada and Atsushi Yoshimura Abstract Background: Pneumoperitoneum commonly occurs as a result of a viscus perforation and usually presents with peritoneal signs requiring emergent laparotomy. Spontaneous pneumoperitoneum is a rare condition characterized by intraperitoneal gas with no clear etiology. Case presentation: We herein report a case in which conservative treatment was achieved for an 83-year-old male patient with spontaneous pneumoperitoneum that probably occurred due to duodenal diverticulosis. He had stable vital signs and slight epigastric discomfort without any other signs of peritonitis. A chest radiograph and computed tomography showed that a large amount of free gas extended into the upper abdominal cavity. Esophagogastroduodenoscopy showed duodenal diverticulosis but no perforation of the upper gastrointestinal tract. He was diagnosed with spontaneous pneumoperitoneum, and conservative treatment was selected. His medical course was uneventful, and pneumoperitoneum disappeared after 6 months. Conclusion: In the management of spontaneous pneumoperitoneum, recognition of this rare condition and an accurate diagnosis based on symptoms and clinical imaging might contribute to reducing the performance of unnecessary laparotomy. However, in uncertain cases with peritoneal signs, spontaneous pneumoperitoneum is difficult to differentiate from free air resulting from gastrointestinal perforation and emergency exploratory laparotomy should be considered for these patients. Keywords: Spontaneous pneumoperitoneum, Duodenal diverticulosis, Conservative management Background therapy. We also discuss the possible etiology and clin- Pneumoperitoneum is caused by perforation of intraperito- ical issues in the management of this rare condition. -

Paraesophageal Hernia
Paraesophageal Hernia a, b Dmitry Oleynikov, MD *, Jennifer M. Jolley, MD KEYWORDS Hiatal Paraesophageal Nissen fundoplication Hernia Laparoscopic KEY POINTS A paraesophageal hernia is a common diagnosis with surgery as the mainstay of treatment. Accurate arrangement of ports for triangulation of the working space is important. The key steps in paraesophageal hernia repair are reduction of the hernia sac, complete dissection of both crura and the gastroesophageal junction, reapproximation of the hiatus, and esophageal lengthening to achieve at least 3 cm of intra-abdominal esophagus. On-lay mesh with tension-free reapproximation of the hiatus. Anti-reflux procedure is appropriate to restore lower esophageal sphincter (LES) competency. INTRODUCTION Hiatal hernias were first described by Henry Ingersoll Bowditch in Boston in 1853 and then further classified into 3 types by the Swedish radiologist, Ake Akerlund, in 1926.1,2 In general, a hiatal hernia is characterized by enlargement of the space be- tween the diaphragmatic crura, allowing the stomach and other abdominal viscera to protrude into the mediastinum. The cause of hiatal defects is related to increased intra-abdominal pressure causing a transdiaphragmatic pressure gradient between the thoracic and abdominal cavities at the gastroesophageal junction (GEJ).3 This pressure gradient results in weakening of the phrenoesophageal membrane and widening of the diaphragmatic hiatus aperture. Conditions that are associated with increased intra-abdominal pressure are those linked -
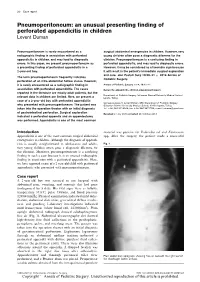
Pneumoperitoneum: an Unusual Presenting Finding of Perforated Appendicitis in Children Levent Duman
20 Case report Pneumoperitoneum: an unusual presenting finding of perforated appendicitis in children Levent Duman Pneumoperitoneum is rarely encountered as a surgical abdominal emergencies in children. However, very radiographic finding in association with perforated young children often pose a diagnostic dilemma for the appendicitis in children, and may lead to diagnostic clinician. Pneumoperitoneum is a confusing finding in errors. In this paper, we present pneumoperitoneum as perforated appendicitis, and may lead to diagnostic errors. a presenting finding of perforated appendicitis in a However, it may be considered as a favorable sign because 2-year-old boy. it will result in the patient’s immediate surgical exploration and cure. Ann Pediatr Surg 10:20–21 c 2014 Annals of The term pneumoperitoneum frequently indicates Pediatric Surgery. perforation of an intra-abdominal hollow viscus. However, it is rarely encountered as a radiographic finding in Annals of Pediatric Surgery 2014, 10:20–21 association with perforated appendicitis. The cases Keywords: appendicitis, children, pneumoperitoneum reported in the literature are mostly adult patients, but the Department of Pediatric Surgery, Su¨leyman Demirel University Medical School, relevant data in children are limited. Here, we present a Isparta, Turkey case of a 2-year-old boy with perforated appendicitis Correspondence to Levent Duman, MD, Department of Pediatric Surgery, who presented with pneumoperitoneum. The patient was Su¨leyman Demirel University Medical School, 32260 Isparta, Turkey taken into the operation theater with an initial diagnosis Tel: + 90 246 2119249; fax: + 90 246 2371758; e-mail: [email protected] of gastrointestinal perforation. Surgical exploration Received 21 July 2013 accepted 26 October 2013 indicated a perforated appendix and an appendectomy was performed. -

Femoral Hernia Causing Pneumoperitoneum
Postgraduate Medical Journal (1986) 62, 675-676 Postgrad Med J: first published as 10.1136/pgmj.62.729.675 on 1 July 1986. Downloaded from Femoral hernia causing pneumoperitoneum H.A. King and P.S. Boulter St Luke's Hospital, Warren Road, Guildford, Surrey GUI 3NT, UK. Summary: Richter's hernia, in which only a portion ofthe circumference ofthe intestine lies within the sac, is a common complication offemoral hernia. This case report is ofa 39 year old female who presented with apneumoperitoneum and was found at laparotomy to have a right femoral Richter's hernia containing a knuckle of perforated small bowel. This is a previously unreported presentation of femoral hernia. Introduction Richter's hernia, in which only a portion of the circumference of the intestine lies within the sac, is a common complication of femoral hernia. The bowel can strangulate and perforate but pneumoperitoneum has never been reported as a complication. Case report A 39 year old female presented with a 10-day history of copyright. generalized abdominal discomfort associated with bile stained vomiting and gross abdominal distension. Her bowels had opened daily. There was no history of previous episodes, dyspepsia or alteration in bowel habit. She had had no previous operations and was otherwise well. On examination, she was dehydrated and febrile (37.5'C), pulse rate 90/min. The abdomen was grossly http://pmj.bmj.com/ distended and tympanitic, diffusely tender but without guarding or rebound tenderness. The hernial orifices were intact. Bowel sounds were quiet and not obstruc- tive. Rectal examination demonstrated only tender- ness anteriorly. -

Management of Complications After Paraesophageal Hernia Repair
10 Review Article Page 1 of 10 Management of complications after paraesophageal hernia repair Abraham J. Botha, Francesco Di Maggio Department of Upper GI Surgery, St Thomas Hospital, Guys and St Thomas NHS Foundation Trust, London, United Kingdom Contributions: (I) Conception and design: AJ Botha; (II) Administrative support: None; (III) Provision of study materials or patients: None; (IV) Collection and assembly of data: All authors; (V) Data analysis and interpretation: None; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Abraham J. Botha. Consultant Upper Gastro-intestinal surgeon, Department of Surgery, St Thomas Hospital. Westminster Bridge Road, SE1 7EH London, United Kingdom. Email: [email protected]. Abstract: Laparoscopic paraesophageal hernia (PEH) repair can be performed safely in expert hands. However, it is a complex operation carrying significant risk of peri-operative morbidity and mortality. Careful intra-operative correctional techniques and prompt return to theatre for early post-operative complications result in a satisfactory outcome. Capnothorax and pneumothorax should be dealt with immediately by lowering insufflation pressure, aspiration and drain placement. Hemodynamic instability from cardiovascular injury or bleeding mandates an early return to theatre. Intra-operative perforation of the esophagus or stomach is best avoided, but it can be successfully repaired. Early acute dysphagia warrants a return to theatre for correction while delayed dysphagia can in some patients be treated by dilatation. Asymptomatic hiatus hernia recurrence does not require surgery, but symptomatic and complicated hernias can be re-repaired. Some life-threatening complications such as acute gastric dilatation, aortic fistula, gastric necrosis and perforation can occur months, and even years, after the procedure. -

Spontaneous Pneumoperitoneum Due to Intra-Abdominal Drain One Week Postprocedure
Case Report DOI: 10.7860/IJARS/2021/46874:2649 Spontaneous Pneumoperitoneum due Surgery Section to Intra-abdominal Drain- A Case Report HIMANGSU SARMA1, GAYATHRI GOPALKRISHNAN2, ASHWIN IKUMAR KUDARI3 ABSTRACT Pneumoperitoneum is most commonly caused by hollow viscus perforation which requires an emergency surgical intervention. However, this is not always the case. Pneumoperitoneum which is not due to hollow viscus perforation is called Spontaneous or “non-surgical” pneumoperitoneum. Rarely, it can present with peritonitis but non-surgical pneumoperitoneum usually follows a benign course and can be managed conservatively. A case of 53-year-old male, non-smoker, non-alcoholic, hypertensive known to have decompensated chronic liver disease, since one year with portal hypertension and ascites was presented. The patient underwent uneventful laparoscopic cholecystectomy for acute calculous cholecystitis and developed spontaneous pneumoperitoneum due to intra-abdominal drain one week postprocedure. Pneumoperitoneum was successfully managed conservatively. A thorough history, physical examination and imaging are crucial in identifying patients with non-surgical pneumoperitoneum and to prevent unnecessary laparotomies. In present case, pneumoperitoneum was due to intra-abdominal drain which resolved after removing the drain. So, it is of utmost importance to rule out non-surgical causes of pnemoperitoneum, especially in surgeries where drain are kept in-situ. Keywords: Computerized tomography, Decompensated liver disease, Non-surgical pneumoperitoneum, Secondary bacterial peritonitis CASE REPORT Clinically, patient was comfortable, vitally stable, and ambulatory A 53-year-old male, non-smoker, non-alcoholic, hypertensive with normal oral intake and bowel habits. The patient did known to have decompensated chronic liver disease since one year not complain of any fever and chills. -

Spontaneous Pneumoperitoneum and Intraperitoneal Free
Postgrad MedJ3 1997; 73: 531 - 537 C The Fellowship of Postgraduate Medicine, 1997 Medical emergencies Postgrad Med J: first published as 10.1136/pgmj.73.863.531 on 1 September 1997. Downloaded from Spontaneous pneumoperitoneum and other nonsurgical causes of intraperitoneal free gas Summary NMA Williams, DFL Watkin Intraperitoneal free gas seen radi- ologically as air under the dia- phragm nearly always indicates a The term pneumoperitoneum is used to describe the presence of free gas within perforated abdominal viscus that the peritoneal cavity but outside the viscera. In the majority of cases (>90%) it is requires surgical intervention. the result of perforation of an intra-abdominal viscus. Generally, prompt Rarely, however, the presence of surgical intervention is required in these patients to reduce the degree and a pneumoperitoneum may not magnitude of enteric contamination within the peritoneal cavity. Consequently, indicate an intra-abdominal per- the presence of intraperitoneal free gas usually mandates surgical referral and foration and thus may not require often results in an emergency laparotomy. There is, however, a subgroup of laparotomy. Such a situation is patients with pneumoperitoneum in whom there are no positive findings. These termed spontaneous or nonsurgi- patients are classified as having 'spontaneous',"2 'misleading',3 or 'nonsurgi- cal pneumoperitoneum. In this cal'4'5 pneumoperitoneum. In this review we explore various aetiological review, we explore the aetiological mechanisms of the appearance of free intraperitoneal -

Free Intestinal Perforation in Children with Crohn's Disease
Journal of Pediatric Surgery Case Reports 32 (2018) 5–10 Contents lists available at ScienceDirect Journal of Pediatric Surgery Case Reports journal homepage: www.elsevier.com/locate/epsc Free intestinal perforation in children with Crohn's disease T ∗ Mila Kolara, , Mercedes Pilkingtona, Andrea Winthropa, Hugh MacDonalda, Christopher Justinichb, Donald Soboleskic, Lloyd Slyc, David Hurlbutd a Department of Surgery, Queen's University, Kingston, Ontario, Canada b Department of Pediatrics, Queen's University, Kingston, Ontario, Canada c Department of Radiology, Queen's University, Kingston, Ontario, Canada d Department of Pathology and Molecular Medicine, Queen's University, Kingston, Ontario, Canada ARTICLE INFO ABSTRACT Keywords: Background: Free intestinal perforation in children with Crohn's disease (CD) is a rare, but serious complication Free intraperitoneal perforation that requires urgent surgical management. The incidence, contributing risk factors, diagnostic workup, and Pediatric Crohn's disease management strategies for these complex pediatric patients are not well established. Methods: We present a recent case of free intestinal perforation in a patient with CD. In addition, a systematic review of the literature was conducted by searching the PubMed, Embase, Ovid, Scopus and Cochrane databases. Two authors independently extracted data, reviewed the abstracts, and assessed them for inclusion in the review. Results: The literature review identified 21 pediatric patients documented in 14 publications; including our case, there are a total of 22 pediatric patients reported. The majority of patients presented with features of peritonitis. Perforation occurred early in the disease course (median 6.5 months), and was most commonly a single per- foration in the ileum with active Crohn's disease (82%). -

Artificial Pneumoperitoneum in the Diagnosis and Treatment of Hiatus Hernia N
Postgrad Med J: first published as 10.1136/pgmj.42.494.765 on 1 December 1966. Downloaded from POSTGRAD. MED. J. (1966), 42, 765. ARTIFICIAL PNEUMOPERITONEUM IN THE DIAGNOSIS AND TREATMENT OF HIATUS HERNIA N. GEFFEN, M.B., Ch.B., D.M.R.D.,* B. MAISEL, M.D. Departments of Radiology and Surgery, New York Hospital -Cornell Medical Center, New York THE ANATOMY and physiology of the distal oesopha- extent of herniation, but after studying 300 cases of gus is well established and the radiological boundar- hiatus hernia by cinefluorography, Campeti, Geffen, ies of hiatus hernia are well defined so that Charles and Watson (1962), believed that the uncertainty in diagnosis is now unnecessary. symptoms were related to the tone of the lower Nevertheless, correlation between the radiological oesophageal sphincter. If the sphincter was hypo- evidence of hiatus hernia and the symtomatology is tonic and overcome, oesophageal reflux would incomplete. Large hernias may exist with minimal occur and this produced symptoms. If the sphincter or no symptoms; conversely, severe symptoms often was hypertonic then dysphagia could result. Such accompany minor radiological changes. a classification, however, though functionally The true incidence of hiatus hernia is unknown, useful, presupposed that the symptoms were all and varies with the techniques employed and in the due to oesophageal reflux and this is clearly an Protected by copyright. selection of patients. The radiological incidence over-simplification. Symptoms may also result from varies from 1 % to 20% (Kirkland and Hodgson, congestive, ulcerative and inflammatory changes in 1947), to 73.3 % (Schatzki, 1932). Edmonds the oesophagus or gastric loculus, early stricture (1957) reports an incidence of 3.3%, Hafter (1958). -

Diseases of the Peritoneum and Retroperitoneum
gastrointestinal tract and abdomen 2 DISEASES OF THE PERITONEUM AND RETROPERITONEUM Amanda K. Arrington, MD, and Joseph Kim, MD Anatomy and Physiology: Peritoneum transverse mesocolon, on the other hand, is the mesentery of the transverse colon and suspends this structure from anatomy the posterior abdominal wall. The root of the transverse The word peritoneum is derived from the Greek terms peri mesocolon extends across the descending duodenum and (“around”) and tonos (“stretching”). The peritoneum, which the head of the pancreas and continues along the inferior lines the innermost surface of the abdominal wall and the border of the body and tail of the pancreas. The transverse majority of the abdominal organs, consists of a layer of mesocolon is continuous with the duodenocolic ligament on dense stroma covered on its inner surface by a single sheet the right and with the phrenicocolic and splenorenal liga- of mesothelial cells. In men, the peritoneum is completely ments on the left. Finally, the sigmoid mesocolon attaches enclosed, whereas in women, the peritoneum is open to the the sigmoid colon to the posterior pelvic wall. This mesen- exterior only at the ostia of the fallopian tubes. The perito- tery, which has an inverted V-shape confi guration, with its neum is divided into two components: the parietal and the apex lying anterior to the bifurcation of the left common ilia c visceral peritoneum [see Figure 1]. The parietal peritoneum artery, contains both sigmoid and hemorrhoidal vessels, covers the innermost surface of the abdominal walls, the lymph nodes, nerves, and abundant fat tissue.3 inferior surface of the diaphragm, and the pelvis. -

Guidelines for the Management of Hiatal Hernia
SAGES Society of American Gastrointestinal and Endoscopic Surgeons http://www.sages.org Guidelines for the Management of Hiatal Hernia Geoffrey P Kohn MBBS(Hons) MSurg FRACS, Raymond R Price MD FACS, Steven R Demeester MD FACS, Joerg Zehetner MD, Oliver J Muensterer MD, Ziad T Awad MD FACS, Sumeet K Mittal MD FACS, William S Richardson MD FACS, Dimitrios Stefanidis MD PhD FACS, Robert D Fanelli MD FACS and the SAGES Guidelines Committee Preamble The guidelines for the management of hiatal hernia are a series of systematically developed statements to assist physicians’ and patients’ decisions about the appropriate use of laparoscopic surgery for hiatal hernia. The statements included in this guideline are the product of a systematic review of published literature on the topic, and the recommendations are explicitly linked to the supporting evidence. The strengths and weaknesses of the available evidence are highlighted and expert opinion sought where the evidence is lacking. Disclaimer Guidelines for clinical practice are intended to indicate preferable approaches to medical problems as established by experts in the field. These recommendations will be based on existing data or a consensus of expert opinion when little or no data are available. Guidelines are applicable to all physicians who address the clinical problem(s) without regard to specialty training or interests, and are intended to indicate the preferable, but not necessarily the only acceptable approaches due to the complexity of the healthcare environment. Guidelines are intended to be flexible. Given the wide range of specifics in any health care problem, the surgeon must always choose the course best suited to the individual patient and the variables in existence at the moment of decision.