Role for Membrane Fluidity in Ethanol-Induced Oxidative Stress of Primary Rat Hepatocytes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 11 CORINTHIAN COLLEGES, INC., Et Al. Case
Case 15-10952-KJC Doc 712 Filed 08/05/15 Page 1 of 2014 IN THE UNITED STATES BANKRUPTCY COURT FOR THE DISTRICT OF DELAWARE In re: Chapter 11 CORINTHIAN COLLEGES, INC., et al.1 Case No. 15-10952-CSS Debtor. AFFIDAVIT OF SERVICE STATE OF CALIFORNIA } } ss.: COUNTY OF LOS ANGELES } SCOTT M. EWING, being duly sworn, deposes and says: 1. I am employed by Rust Consulting/Omni Bankruptcy, located at 5955 DeSoto Avenue, Suite 100, Woodland Hills, CA 91367. I am over the age of eighteen years and am not a party to the above-captioned action. 2. On July 30, 2015, I caused to be served the: a) Notice of (I) Deadline for Casting Votes to Accept or Reject the Debtors’ Plan of Liquidation, (II) The Hearing to Consider Confirmation of the Combined Plan and Disclosure Statement and (III) Certain Related Matters, (the “Confirmation Hearing Notice”), b) Debtors’ Second Amended and Modified Combined Disclosure Statement and Chapter 11 Plan of Liquidation, (the “Combined Disclosure Statement/Plan”), c) Class 1 Ballot for Accepting or Rejecting Debtors’ Chapter 11 Plan of Liquidation, (the “Class 1 Ballot”), d) Class 4 Ballot for Accepting or Rejecting Debtors’ Chapter 11 Plan of Liquidation, (the “Class 4 Ballot”), e) Class 5 Ballot for Accepting or Rejecting Debtors’ Chapter 11 Plan of Liquidation, (the “Class 5 Ballot”), f) Class 4 Letter from Brown Rudnick LLP, (the “Class 4 Letter”), ____________________________________________________________________________________________________________________________________________________________________________________________________________ 1 The Debtors in these cases, along with the last four digits of each Debtor’s federal tax identification number, are: Corinthian Colleges, Inc. -

Teacher's Notes Subbie and His Mate
Teacher’s Notes Subbie and his mate Author: Corinne Fenton Illustrator: Mark Wilson Publisher: Ford Street Publishing 2022 ‘Every morning Subbie would wait at the gate for Graham, and every morning they would talk. Subbie’s eyes watched Graham wherever he went and, whenever he could, he followed.’ About the Author Corinne Fenton writes picture books about animals whose lives have touched our hearts. Many of her picture books have been shortlisted or won awards and three have been chosen as themes for the Myer Christmas windows. Corinne has spent time as the Assistant Regional Advisor for the Society of Children’s Books Writers and Illustrators in Australia and as a judge for the Dorothea Mackellar poetry competition. In her picture books, Corinne introduces us to animals who have a special place in Australian history. This is one of them. Web: https://corinnefenton.com/ About the Illustrator Mark Wilson is a multiple award-winning author/illustrator with 23 books in print worldwide in 14 languages. Mark grew up with a love of comics, drawing, rock music, Australian history and endangered species - exploring most of these themes through his writing, illustrating, paintings and workshops. Mark has won seven Australian National Fine Art awards, five Whitley Awards for children’s literature, nine CBCA Awards, four Wilderness Society Awards and the 3rd C. J. Picture Book International Award. Mark was also presented with the 2011 Dromkeen Medal for Services to Children’s Literature. Mark hopes his books and workshops encourage students to study their own family and local history, as well as native species and conservation. -

Historical Painting Techniques, Materials, and Studio Practice
Historical Painting Techniques, Materials, and Studio Practice PUBLICATIONS COORDINATION: Dinah Berland EDITING & PRODUCTION COORDINATION: Corinne Lightweaver EDITORIAL CONSULTATION: Jo Hill COVER DESIGN: Jackie Gallagher-Lange PRODUCTION & PRINTING: Allen Press, Inc., Lawrence, Kansas SYMPOSIUM ORGANIZERS: Erma Hermens, Art History Institute of the University of Leiden Marja Peek, Central Research Laboratory for Objects of Art and Science, Amsterdam © 1995 by The J. Paul Getty Trust All rights reserved Printed in the United States of America ISBN 0-89236-322-3 The Getty Conservation Institute is committed to the preservation of cultural heritage worldwide. The Institute seeks to advance scientiRc knowledge and professional practice and to raise public awareness of conservation. Through research, training, documentation, exchange of information, and ReId projects, the Institute addresses issues related to the conservation of museum objects and archival collections, archaeological monuments and sites, and historic bUildings and cities. The Institute is an operating program of the J. Paul Getty Trust. COVER ILLUSTRATION Gherardo Cibo, "Colchico," folio 17r of Herbarium, ca. 1570. Courtesy of the British Library. FRONTISPIECE Detail from Jan Baptiste Collaert, Color Olivi, 1566-1628. After Johannes Stradanus. Courtesy of the Rijksmuseum-Stichting, Amsterdam. Library of Congress Cataloguing-in-Publication Data Historical painting techniques, materials, and studio practice : preprints of a symposium [held at] University of Leiden, the Netherlands, 26-29 June 1995/ edited by Arie Wallert, Erma Hermens, and Marja Peek. p. cm. Includes bibliographical references. ISBN 0-89236-322-3 (pbk.) 1. Painting-Techniques-Congresses. 2. Artists' materials- -Congresses. 3. Polychromy-Congresses. I. Wallert, Arie, 1950- II. Hermens, Erma, 1958- . III. Peek, Marja, 1961- ND1500.H57 1995 751' .09-dc20 95-9805 CIP Second printing 1996 iv Contents vii Foreword viii Preface 1 Leslie A. -

Eu Whoiswho Official Directory of the European Union
EUROPEAN UNION EU WHOISWHO OFFICIAL DIRECTORY OF THE EUROPEAN UNION EUROPEAN COMMISSION 16/09/2021 Managed by the Publications Office © European Union, 2021 FOP engine ver:20180220 - Content: - merge of files"Commission_root.xml", "The_College.XML1.5.xml", "temp/CRF_COM_CABINETS.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_SG.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/ CRF_COM_SJ.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_COMMU.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_IDEA.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_BUDG.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/ CRF_COM_HR.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_DIGIT.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_IAS.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_OLAF.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/ CRF_COM_ECFIN.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_GROW.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_DEFIS.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_COMP.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/ CRF_COM_EMPL.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_AGRI.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_MOVE.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_ENER.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/ CRF_COM_ENV.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_CLIMA.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_RTD.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/CRF_COM_CNECT.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", "temp/ CRF_COM_JRC.RNS.FX.TRAD.DPO.dated.XML1.5.ANN.xml", -
2021 Lyon County Fair 4-H and Open Class Schedule and Grandstand Events
2021 Lyon County Fair 4-H and Open Class Schedule and Grandstand Events 1 THURSDAY July All 4-H pre-entries due to the Extension Office 15 THURSDAY July 5:00 p.m. Scripts Due to Extension Office via email to [email protected] 29 Thursday July 6:30 p.m. Friends of 4-H Picnic – Anderson Building 30 Friday July 5:00 p.m. Dog, Hand Pet, and Cat Shows – Anderson Building 31 Saturday July 8:00 a.m. 4-H Clothing Construction judging begins – Anderson Building Modeling will follow Conference Judging 9:00 a.m. 4-H Boys Buymanship, 4-H Girls Buymanship conference judging begins – Anderson Building Modeling will follow Conference Judging 1:30 p.m. 4-H Photography, 4-H Home Environment, 4-H Entomology, 4-H Rocketry, 4-H Forestry, 4-H Fiber Arts, 4-H Electric, 4-H Reading, 4-H Leadership & 4-H Other Projects – Anderson Building 4-H Geology (TBA) 6:00 p.m. Public Fashion Revue – Anderson Building 1 Sunday Aug. 8:00 a.m. Horse Show – Grandstands ust 2 MONDAY Aug. 5:00 p.m. Fairgrounds Cleanup and Anderson Building cleaning – Check with Club Leader for assignments 3 TUESDAY Aug. 5:00 -6:00 p.m. 4-H Exhibits entered in Anderson Building except those being conference judged 5:30 p.m. 4-H Visual Arts, 4-H Metals, 4-H Woodworking 4 Wednesday Aug. 5:30 p.m. 4-H Foods, 4-H Floriculture & Horticulture, & 4-H Crops 5 Thursday Aug. 11:30 a.m. – 1:30 p.m. -
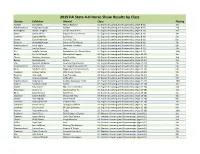
2019 State Horse Show Results by Class and County
2019 PA State 4-H Horse Show Results by Class County Exhibitor Animal Class Placing Fayette Joely Miller Million Reasons 01: English Grooming and Showmanship, (Ages 8-11) 1st Westmoreland Abby McCullough Harllee 01: English Grooming and Showmanship, (Ages 8-11) 2nd Huntingdon Hannah Feagley Secure Your Assets 01: English Grooming and Showmanship, (Ages 8-11) 3rd Potter Savannah Kio Peppers Precious Penny 01: English Grooming and Showmanship, (Ages 8-11) 4th Crawford Sophie Wehrle Zip O Cool 01: English Grooming and Showmanship, (Ages 8-11) 5th Snyder Gavyn Heimbach I Can Pass Too 01: English Grooming and Showmanship, (Ages 8-11) 6th Dauphin Charlotte Duncan Dancin In The Weeds 01: English Grooming and Showmanship, (Ages 8-11) 7th Westmoreland Anna Zeglin Attractive Invitation 01: English Grooming and Showmanship, (Ages 8-11) 8th Warren Emelyn Moore skip 01: English Grooming and Showmanship, (Ages 8-11) 9th Erie Isabella Cannata Romandaros Sirs Painted Bear 01: English Grooming and Showmanship, (Ages 8-11) 10th Berks Caitlin Diffendal Miracles Do Happen 02: English Grooming and Showmanship, (Ages 12-14) 1st Clinton Madelyn Hendricks Invy The Ride 02: English Grooming and Showmanship, (Ages 12-14) 2nd Beaver Charity Tellish Kuhlua 02: English Grooming and Showmanship, (Ages 12-14) 3rd Erie Madison Anderson One Smoking Maverick 02: English Grooming and Showmanship, (Ages 12-14) 4th Westmoreland Kaitlynn Lebo I've Tango'd My Socks Off 02: English Grooming and Showmanship, (Ages 12-14) 5th Berks Elizabeth Jones Magic Sweet Princes Beauty 02: -

2017 State Horse Show Results by Class
2017 PA 4-H Horse Show Results by Class Place Exhibitor Town Horse County English Grooming & Showmanship, 8-11 1 Hannah FeagleyPetersburg Secure Your Assets Huntingdon 2 Abby McCulloughManor Shieks Special Te Westmoreland 3 Adyson FranzMoon Township Echo Beaver 4 Abby SpinoGreensburg One Hot Capachino Westmoreland 5 Coveyn BearBenton Deon Columbia 6 Emma FuchsVolant Art I A Lark Lawrence 7 Ashlee HackPalmyra Hunting with Faith Dauphin 8 Yanina OteroPalmyra Hopeful Hobby Dauphin 9 Julia DiNapoliWalnutport Penny in Your Pocket Northampton 10 Alycia SzyskoWarrington Bullseye Bucks English Grooming & Showmanship, 12-14 1 Evalee BinnsBrownsville So Hot Im Krymsun Washington 2 Brook BrehmWindber Envious Version Somerset 3 Caiden BathkeEllwood City Knights Miss Swiss Lawrence 4 Madelyn HendricksJersey Shore Invy The Ride Clinton 5 Sarah FisherGeorgetown Pardons Sonny Beaver 6 Emilee HeverlyMill Hall Lil Bonanza Leaguer Clinton 7 Malaina MacostaDaisytown Jutti Westmoreland 8 Nadia SlishHonesdale Wildfire Wayne 9 Jenna TysonPottstown My Tea Impact Berks 10 Rylan FaganZion Grove CSH Porcelain Doll Columbia English Grooming & Showmanship, 15-18 1 Alexus HartmanMcClure Just Another Detail Mifflin 2 Laura LanagerFrenchville Zippos Sweet C Clearfield 3 Katharine HendricksJersey Shore A Good Premonition Clinton 4 Madison HeilveilLansdale Backwoods Boy Bucks 5 Emily MannaHarrisburg Sr Lil Bit of Love Dauphin 6 Cambrie ShortGreensburg Mostly Cloudy Skies Westmoreland 7 Katherine BowserEllwood City Sonnys Bedazzled Lawrence 8 Kyrsten KowalczykFlinton Fire -

SIKURA's FAITH REWARDED by GRADE I EXACTA Attempt to Get Back in the Winner=S Enclosure at Royal Ascot Maclean's Music Sired the GI Woody Stephens S
FRIDAY, JUNE 11, 2021 OBS JUNE CONTINUES WITH SOLID RESULTS SIKURA'S FAITH by Jessica Martini REWARDED BY OCALA, FL - Steady trade continued through the second session of the Ocala Breeders' Sales Company June Sale of 2-Year-Olds GRADE I EXACTA Thursday in Central Florida, with a filly by Nyquist bringing the day's top bid when selling for $420,000 to Gary Hartunian's Rockingham Ranch. The session-topping juvenile was consigned by Eddie Woods. AIt was another good day,@ OBS Director of Sales Tod Wojciechowski said at the close of business Thursday. AWe carried a lot of the momentum that we had yesterday into today and I think it will carry on through tomorrow.@ With two sessions in the books, OBS has sold 380 head for $15,776,500. The two-day average is $41,517 and the median is $18,000. With 106 horses reported not sold, the buy-back rate was 21.8%. Cont. p3 IN TDN EUROPE TODAY Maclean's Music | Hill 'n' Dale O'BRIEN RUNNERS ON ASCOT COMEBACK MISSIONS Joseph O'Brien's Group 1-winning juveniles Thunder Moon by Chris McGrath (Ire) (Zoffany {Ire}) and Pretty Gorgeous (Ire) (Lawman {Fr}) will make their returns at Royal Ascot next week. Click or tap here to It is now a decade since John Sikura was walking through a go straight to TDN Europe. Lexington steakhouse and glimpsed, on a screen over the bar, a bay colt coasting clear of his pursuers with sparks coming from his heels: :21.24, :43.48, 1:07.44. -

Download All Friday
WIN PLACE SHOW Approx.6 Post Time 2:30 p.m. Turf Course JUVENILE TURF SPRINT GRADE II - $1,000,000 GUARANTEED Exacta / Trifecta / Superfecta FOR TWO-YEAR-OLDS Pick 3 (Races 6-7-8) / Daily Double Pick 5 (Races 6-10) / Jackpot Super High Five ODDS OWNER TRAINER JOCKEY ODDS OWNER TRAINER JOCKEY RABBAH BLOODSTOCK, LLC, LESSEE ARCHIE WATSON BREEZE EASY, LLC WESLEY A. WARD (BRIAN R. THOMPSON) (MIKE HALL) 1 Royal Blue, White Ball, White Dots on Sleeves, 9 Yellow, Blue Collar, Yellow 'BE' on Blue Ball, Yellow Royal Blue Cap and Blue Vertically Halved Sleeves, Blue Visor on Yellow Cap MIGHTY GURKHA (IRE) 122 AFTER FIVE 122 B.c.2 Sepoy (AUS) - Royal Debt (GB) Gr/ro.c.2 The Factor - Idle Talk 20-1 B.c.2 Sepoy (AUS) - by Royal Applause (GB) Hollie 6-1 Gr/ro.c.2 The Factor - by Olmodavor Jose Red Bred in IRE by RABBAH BLOODSTOCK LIMITED Doyle Turquoise Bred in MD by MARY E. EPPLER RACING STABLE & A. LEONARD PINEAU Ortiz RYAN C. RITT, JEFFREY H. GASNER JONATHAN WONG KRISTIN BOICE & MARYLOU HOLDEN VALORIE LUND & MARK SPINAZZE 2 Blue, Mirrored Blue 'R' on White Ball, White Blocks on Sleeves, 10 Green, White Collar, White Epaulets, White Circled Horse Emblem and Blue Blocks on White Cap 'LUND,' White Cuffs on Sleeves, Green Cap WINDY CITY RED 122 BODENHEIMER 122 Ch.c.2 Chitu - Gator Hall Dk B/ Br.c.2 Atta Boy Roy - Beautiful Daniele 30-1 Ch.c.2 Chitu - by Graeme Hall Jose 8-1 Dk B/ Br.c.2 Atta Boy Roy - by A.P. -

Chapter 2: Zombies, Gender and World-Ecology: Gothic Narrative in the Work of Ana Lydia Vega and Mayra Montero
Chapter 2: Zombies, Gender and World-Ecology: Gothic Narrative in the Work of Ana Lydia Vega and Mayra Montero Kerstin Oloff The individual, the community, the land, are inextricable in the process of creating history. Landscape is a character in this process. Its deepest meanings need to be understood – Édouard Glissant (1989, 105-6) It is widely accepted that Gothic fears construct ‘a monster out of the traits which ideologies of race, class, gender, sexuality and capital want to disavow’ (Halberstam, 1995, 102). Indeed, much has been written on the Gothic’s inherent relation to racist-patriarchal capitalism, but the role of ‘ecophobia’ within the Gothic has only more recently become a focus of sustained critical attention.1 Historical capitalism has developed through a series of metabolic rifts that have as their ideological complement the nature-society dichotomy (a dichotomy which is also gendered and racialized). Put simply, these ‘rifts’ refer to the increasing alienation of the majority of the population from the means of reproduction – most fundamentally, the land and the body. The zombie is ideal for starting to think through Gothic representations of these rifts (Oloff, 2012). Zombies have become globally recognizable figures because they speak powerfully to the anxieties produced by the commodification of labour: humans are reduced to being bodily vessels for the production of specifically capitalist value (socially necessary labour time). Yet commodification is also fundamentally an ecological process, something that becomes clear if we consider the zombie’s Haitian origins. The zombie has its roots in a paradigmatic moment in the emergence of capitalism: the Caribbean experience of the sugar 1 Ecophobia is defined by Estok as ‘an irrational fear (sometimes, of course, leading to contempt or hatred) of the agency (real or imagined) of nature’ (2013, 74). -

Baffert Hoping Eight Is Great for Mckinzie in Breeders
TUESDAY, OCTOBER 29, 2019 BAFFERT HOPING EIGHT IS VERACITY BRINGS PROVEN TRACK RECORD TO FASIG NOVEMBER By Bill Finley GREAT FOR MCKINZIE IN When attacking the fall mixed sales, the key to future success lies in the ability to properly measure risk versus reward. That=s BREEDERS= CUP CLASSIC 36 why the eight-year-old mare Veracity (Distorted Humor), in foal to Justify, figures to bring a healthy sum at the Fasig-Tipton November Night of the Stars Nov. 5. The risk is minimal and the rewards are all but guaranteed. Veracity has had two foals that have been put up for auction, both fillies by Medaglia d=Oro. This year, her yearling sold for $900,000 at the Saratoga Sale and was purchased by Claiborne Farm. In 2018, Lael Stable spent $1 million on her first yearling, also purchased at Saratoga. AHer family is very active now,@ said consignor Shack Parrish, the president of Indian Creek. AFor her first two foals to sell for what they did speaks volumes. She=s in foal to Justify, her family is very alive and her first two foals sold for what they did. That makes her the whole package.@ Cont. p10 IN TDN EUROPE TODAY McKinzie, shown here breezing Monday at Santa Anita, was pegged the 3-1 favorite for Saturday=s GI Breeders= Cup Classic | Horsephotos MAGICAL SPIKES TEMP, OUT OF BC Champion Magical (Ire) (Galileo {Ire}) spiked a temperature prior to by Alan Carasso shipping to Santa Anita for the Breeders’ Cup and has instead been Hall of Fame conditioner Bob Baffert will go down as one of retired. -

Racing at Its Best: the Personal Ensign
MONDAY, AUGUST 26, 2019 RACING AT ITS BEST: SPENDTHRIFT ACQUIRES BREEDING RIGHTS TO MITOLE THE PERSONAL ENSIGN B. Wayne Hughes=s Spendthrift Farm has acquired the breeding rights to Bill and Corinne Heiligbrodt=s multiple Grade I-winning sprinter Mitole (Eskendereya--Indian Miss, by Indian Charlie). While Mitole=s fee will be subject to change pending future race results, the farm is offering breeders the opportunity to lock in at a fee of $20,000 S&N for 2020. AThe term >brilliance= gets used a lot in this business, but there aren=t many horses in recent history that have shown as much consistent brilliance as Mitole,@ said Spendthrift general manager Ned Toffey. AHis Met Mile sticks in my head. That was both the field of the year and race of the year so far, and Mitole showed just how brilliantly fast and classy he is in that performance. On top of having rare ability, he=s an extremely good-looking animal.@ Cont. p6 Midnight Bisou (outside) outbattles Elate | Sarah Andrew IN TDN EUROPE TODAY The Week in Review, By Bill Finley BARTON STUD BACK IN PREMIER FRAY It was the day of the GI Runhappy Travers S., and that=s a race A year after offering its first consignment at the Goffs UK that will always command the most attention on the most Premier Yearling Sale, Barton Stud is back with 10 yearlings important day of the Saratoga meet. But the 48,213 fans who this week. Click or tap here to go straight to TDN Europe. packed the stands Saturday saw what will be hard to top when it comes to the best race run this year, the GI Personal Ensign S.