Evaluation of Groundwater Quality in and Around Ariyalur of Tamilnadu Using Geographical Information System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

District Legal Services Authority, Ariyalur (District Court, Ariyalur) Dated: 11.01.2018
DISTRICT LEGAL SERVICES AUTHORITY, ARIYALUR (DISTRICT COURT, ARIYALUR) DATED: 11.01.2018 LIST OF THE CANDIDATES SHORT LISTED FOR THE POST OF JUNIOR ADMINISTRATIVE ASSISTANT (BC-CATEGORY) TO ATTEND THE INTERVIEW AT DISTRICT LEGAL SERVICES AUTHORITY, ARIYALUR (DISTRICT COURT, ARIYALUR) Sl. Application Name and Address Date & Time No. No. D. Antoni George S/o Durai Raj 1 Employment 3/ 209 North Street 30.01.2018 (ARD2014M00008452) Sembiyakudi Village 9.00 A.M. Kulamanickam Post Ariyalur TK & Dist 621 722 2 S. Selvaraju S/o Subbrayan 30.01.2018 Employment Poyyur (ARD1993M00019772) Karuppursenapathi Post 9.00 A.M. Ariyalur Dist 621 707 3 M. Paramasivam S/o Muthusamy Udayar 30.01.2018 Employment Middle Street (ARD1989M00013257) Rayampuram Post 9.00 A.M. Ariyalur TK & Dist 621 718 4 S. Pattu W/o Ravichandra bose 30.01.2018 Employment 72/8 Periyar Nagar (ARD2004F00000537) First Street 9.00 A.M. Ariyalur Dist 621 704 5 S. Karunanithi S/o Santhalingam 23/2nd Street 30.01.2018 Employment Sengunathapuram Post (ARD1994M00051423) 9.00 A.M. Udayarpalayam TK Ariyalur Dist 621 802 6 N.Govardhan, S/o.P.Nagaraj, 20, 3 B4/1, 30.01.2018 JAA-02 Thendral Nagar East Street, 9.00 A.M. Bodi Nayakkanur – PO Theni District 7 P.Raj, S/o. Pavunraj 30.01.2018 JAA-03 No.25, south street Elakkurichi– PO 9.00 A.M. Ariyalur. 8 A.Latha D/o. Annavi No.1/50 Maniya Nagar Ettarai – PO 30.01.2018 JAA-06 Kulumani – via, 9.00 A.M. Trichy 639 103. 9 M.Senthilmurugan S/o. -

Download 7.98 MB
Project Number: 52041-002 August 2021 Integrated High Impact Innovation in Sustainable Energy Technology Prefeasibility Analysis for Carbon Capture, Utilization and Storage (Subproject 2) Prepared by BCS Baliga, Ramesh Bhujade, Subhamoy Kar, Guido Magneschi, V Karthi Velan, Dewika Wattal, and Jun Zhang For ADB Energy Sector Group This the Government cannot be held liable for its contents. Project Number: 52041-002 Integrated High Impact Innovation in Sustainable Energy Technology - Prefeasibility Analysis for Carbon Capture, Utilization and Storage (Subproject 2) Prefeasibility Study on Carbon Capture and Utilization in Cement Industry of India August 2021 1 ABBREVIATIONS AND NOTES ABBREVIATIONS ADB Asian Development Bank CCS Carbon Capture and Storage CCU Carbon Capture and Utilization CCUS Carbon Capture Utilization and Storage CAPEX Capital expenditure CIF Cost, Insurance and Freight CO2 Carbon dioxide CSI Cement Sustainability Initiative CUP CO2 Utilization Plant DAC Direct air capture DBL Dalmia Bharat Limited DCBL Dalmia Cement (Bharat) Limited EA Executing Agency EOR Enhanced oil Recovery EGR Enhanced gas Recovery ECBM Enhanced coal bed methane FOB Free on Board FY Financial Year H2 Hydrogen IA Implementing Agency INDC Intended Nationally Determined Contributions IRR Internal Rate of Return MCA Multi Criteria Analysis MIRR Modified Internal Rate of Return MTPA Million Tonnes Per Annum NPV Net Present Value OPEX Operating expenditure SPV Special Purpose Vehicle 2 TA Technical Assistance tpa tonnes per annum TRL Technology readiness level VGF Viability Gap Funding WACC Weighted Average Cost of Capital WDV Written Down Value NOTES (i) The fiscal year (FY) of the Government and its agencies ends on March 31. (ii)In this report, "$" refers to US dollars, unless otherwise stated. -

Urban Development in Ariyalur District, Using Remote Sensing and Geographical Information System (Gis)
R.Vasanthi et al Int. Journal of Engineering Research and Applications www.ijera.com ISSN : 2248-9622, Vol. 4, Issue 1( Version 3), January 2014, pp.280-291 RESEARCH ARTICLE OPEN ACCESS Urban Development in Ariyalur District, Using Remote Sensing and Geographical Information System (Gis) 1 R.Vasanthi , 1R. Baskaran, and 2 G.Vanaraju. 1 Department of Industries and Earth sciences, Tamil University, Thanjavur – 613010, TamilNadu, India 2 Department of Geology, School of Geosciences, Bharathidasan University, Tiruchirappalli – 620024, Tamil Nadu, India Abstract The study is mainly based on visual interpretation of satellite imageries by studying the standard recognition elements such as color, tone, texture, Pattern etc., for the delineation of urban land use of the study area. After the visual interpretation of Satellite imageries direct field checks have been made. The primary data were acquired from the LANDSAT satellite imagery. The supplementary data were generated from the survey of India SOI topographical maps. A base map was prepared using survey of India toposheets having the index of numbers, 58M/4, 7, 8, 11, 12 and 58N/1 on a scale of 1:50,000 as an understanding of this study. Totally 6 toposheets have covered the study area. The geographical features like major road, railway, and drainage system, and elevation information, nature of River, tank, settlements and relevant information were incorporated in the base map. Arc GIS used to integrate the available data sources. LANDSAT MSS (1976), IRS P6-LISS III (2010) satellite data the urban land use classification were attempted. The classification followed here is based on unsupervised classification and interpretation; the data interpreted from the imagery were cross- checked in the field. -

Tamil Nadu Government Gazette Published by Authority
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2018 [Price : Rs. 2.40 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 47] CHENNAI, WEDNESDAY, NOVEMBER 21, 2018 Karthigai 5, Vilambi, Thiruvalluvar Aandu – 2049 Part VI—Section 1 Notifications of interest to the General Public issued by Heads of Departments, Etc. NOTIFICations BY HEADS OF Departments, ETC. CONTENTS Pages. GENERAL NOTIFICationS Variation to the Approved Kumarapalayam Detailed Developmet Plan No. 7 of Coimbatore Local Planning Area .. .. .. .. .. .. .. .. .. 366 JUDICIAL NOTIFICationS Constitution of District Munsif-cum-Judicial Magistrate Court at Sendurai in Ariyalur District .. .. 366-367 Constitution of District Munsif-cum-Judicial Magistrate Court at Athoor in Dindigul District .. 367 Approval of Neendakarai ‘A’ Detailed Development Plan No.2 of Nagercoil Local Planning Area 368 Variations to the Approved Master Plan for the Coimbatore Local Planning Area, etc .. 368-369 Variation to the Approved Master Plan for Kurichi New Town Development Plan .. .. 369-370 [365] DTP—VI-1 (47) 366 TAMIL NADU GOVERNMENT GAZETTE [Part vi—Sec.1 NOTIFICATIONS BY HEADS OF DEPARTMENTS, ETC. GENERAL NOTIFICationS Variation to the Approved Kumarapalayam Detailed Development Plan No. 7 of Coimbatore Local Planning Area. (Roc.No. 5505/2018/DP2) No.VI(1)/412/2018. In exercise of the powers conferred under sub-section (1) of Section 33 of Town and Country Planning Act, 1971. (Act No. 35 of 1972), the Commissioner of Town and Country Planning, in -

DISTRICT SURVEY REPORT for LIMEKANKAR ARIYALUR DISTRICT TAMILNADU STATE (Prepared As Per Gazette Notification S.O.3611 (E) Dated
DISTRICT SURVEY REPORT FOR LIMEKANKAR ARIYALUR DISTRICT TAMILNADU STATE (Prepared as per Gazette Notification S.O.3611 (E) dated 25.07.2018 of Ministry of Environment, Forest and Climate Change) 1 Chapter Content Page No. 1. Introduction 3 2. Overview of Mining Activity in the District 3 3. General Profile of the District 3-7 4. Geology of Ariyalur District 7-11 5. Drainage of Irrigation pattern 12 6. Land Utilisation Pattern in the District: Forest, Agricultural, 12-13 Horticultural, Mining etc., 7. Surface Water and Ground Water scenario of the District 13-19 8. Climate and Rainfall of the District 19 9. Details of Mining Leases in the District 20 10. Details of Royalty or Revenue received in last three years 20 11. Details of Production of Minor Mineral in last three years 20 12. Mineral Map of the District 21 13. List of Letter of Intent (LOI) Holder in the District along with 22-23 its validity 14. Total Mineral Reserve available in the district 24-25 15. Quality/Grade of Mineral available in the district 26 16. Use of Mineral 26 17. Demand and supply of the Mineral in the last three years 26 18. Mining Leases marked on the map of the district 26 19. Details of the area of where there is a cluster of the mining 27 leases 20. Details of Eco-sensitive area 27-28 21. Impact on the environment due to Mining activity 28-30 22. Remedial measures to mitigate the impact of mining on the 30-32 environment 23. Reclamation of the mined out area 32 24. -

District Survey Report for Limekankar Ariyalur District Tamilnadu State
DISTRICT SURVEY REPORT FOR LIMEKANKAR ARIYALUR DISTRICT TAMILNADU STATE (Prepared as per Gazette Notification S.O.3611 (E) dated 25.07.2018 of Ministry of Environment, Forest and Climate Change) 1 Chapter Content Page No. 1. Introduction 3 2. Overview of Mining Activity in the District 3 3. General Profile of the District 3-7 4. Geology of Ariyalur District 7-11 5. Drainage of Irrigation pattern 12 6. Land Utilisation Pattern in the District: Forest, Agricultural, 12-13 Horticultural, Mining etc., 7. Surface Water and Ground Water scenario of the District 13-19 8. Climate and Rainfall of the District 19 9. Details of Mining Leases in the District 20 10. Details of Royalty or Revenue received in last three years 20 11. Details of Production of Minor Mineral in last three years 20 12. Mineral Map of the District 21 13. List of Letter of Intent (LOI) Holder in the District along with 22-23 its validity 14. Total Mineral Reserve available in the district 24-25 15. Quality/Grade of Mineral available in the district 26 16. Use of Mineral 26 17. Demand and supply of the Mineral in the last three years 26 18. Mining Leases marked on the map of the district 26 19. Details of the area of where there is a cluster of the mining 27 leases 20. Details of Eco-sensitive area 27-28 21. Impact on the environment due to Mining activity 28-30 22. Remedial measures to mitigate the impact of mining on the 30-32 environment 23. Reclamation of the mined out area 32 24. -
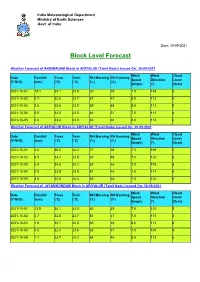
Block Level Forecast
India Meteorological Department Ministry of Earth Sciences Govt. of India Date: 30-09-2021 Block Level Forecast Weather Forecast of ANDIMADAM Block in ARIYALUR (Tamil Nadu) Issued On: 30-09-2021 Wind Wind Cloud Date Rainfall Tmax Tmin RH Morning RH Evening Speed Direction Cover (Y-M-D) (mm) (°C) (°C) (%) (%) (kmph) (°) (Octa) 2021-10-01 18.1 34.1 23.9 80 39 7.0 158 5 2021-10-02 0.7 32.6 23.7 81 47 8.0 113 5 2021-10-03 2.6 33.6 23.9 85 48 8.0 113 8 2021-10-04 0.0 32.5 23.5 81 51 7.0 113 4 2021-10-05 0.8 33.8 24.0 83 46 8.0 113 6 Weather Forecast of ARIYALUR Block in ARIYALUR (Tamil Nadu) Issued On: 30-09-2021 Wind Wind Cloud Date Rainfall Tmax Tmin RH Morning RH Evening Speed Direction Cover (Y-M-D) (mm) (°C) (°C) (%) (%) (kmph) (°) (Octa) 2021-10-01 3.6 35.6 24.2 77 38 7.0 195 5 2021-10-02 6.5 33.4 23.5 80 48 7.0 120 6 2021-10-03 2.4 34.8 24.1 83 46 7.0 108 8 2021-10-04 0.8 33.8 23.8 81 46 7.0 114 4 2021-10-05 3.0 34.8 24.4 82 46 7.0 120 6 Weather Forecast of JAYAMKONDAM Block in ARIYALUR (Tamil Nadu) Issued On: 30-09-2021 Wind Wind Cloud Date Rainfall Tmax Tmin RH Morning RH Evening Speed Direction Cover (Y-M-D) (mm) (°C) (°C) (%) (%) (kmph) (°) (Octa) 2021-10-01 13.5 34.1 24.0 80 39 7.0 120 5 2021-10-02 0.7 32.8 23.7 81 47 7.0 113 5 2021-10-03 1.8 33.7 24.0 85 48 8.0 113 8 2021-10-04 0.0 32.4 23.6 82 51 7.0 105 4 2021-10-05 1.1 33.7 24.2 84 46 8.0 113 6 India Meteorological Department Ministry of Earth Sciences Govt. -

U.Vinothini, 1/65, Cinnakuruvadi, Irulneekki, Mannargudi Tk
Page 1 of 108 CANDIDATE NAME SL.NO APP.NO AND ADDRESS U.VINOTHINI, 1/65, CINNAKURUVADI, IRULNEEKKI, 1 7511 MANNARGUDI TK,- THIRUVARUR-614018 P.SUBRAMANIYAN, S/O.PERIYASAMY, PERUMPANDI VILL, 2 7512 VANCHINAPURAM PO, ARIYALUR-0 T.MUNIYASAMY, 973 ARIYATHIDAL, POOKKOLLAI, ANNALAKRAHARAM, 3 7513 SAKKOTTAI PO, KUMBAKONAM , THANJAVUR-612401. R.ANANDBABU, S/O RAYAPPAN, 15.AROCKIYAMATHA KOVIL 4 7514 2ND CROSS, NANJIKKOTTAI, THANJAVUR-613005 S.KARIKALAN, S/O.SELLAMUTHU, AMIRTHARAYANPET, 5 7515 SOLAMANDEVI PO, UDAYARPALAYAM TK ARIYALUR-612902 P.JAYAKANTHAN, S/O P.PADMANABAN, 4/C VATTIPILLAIYAR KOILST, 6 7516 KUMBAKONAM THANJAVUR-612001 P.PAZHANI, S/O T.PACKIRISAMY 6/65 EAST ST, 7 7517 PORAVACHERI, SIKKAL PO, NAGAPATTINAM-0 S.SELVANAYAGAM, SANNATHI ST 8 7518 KOTTUR &PO MANNARGUDI TK THIRUVARUR- 614708 Page 2 of 108 CANDIDATE NAME SL.NO APP.NO AND ADDRESS JAYARAJ.N, S/O.NAGARAJ MAIN ROAD, 9 7519 THUDARIPETKALIYAPPANALLUR, TARANGAMPADI TK, NAGAPATTINAM- 609301 K.PALANIVEL, S/O KANNIYAN, SETHINIPURAM ROAD ST, 10 7520 VIKIRAPANDIYAM, KUDAVASAL , THIRUVARUR-610107 MURUGAIYAN.K, MAIN ROAD, 11 7521 THANIKOTTAGAM PO, VEDAI, NAGAPATTINAM- 614716 K.PALANIVEL, S/O KANNIYAN, SETHINIPURAM,ROAD ST, 12 7522 VIKIRAPANDIYAM, KUDAVASAL TK, TIRUVARUR DT INDIRAGANDHI R, D/O. RAJAMANIKAM , SOUTH ST, PILLAKKURICHI PO, 13 7523 VARIYENKAVAL SENDURAI TK- ARIYALUR-621806 R.ARIVAZHAGAN, S/O RAJAMANICKAM, MELUR PO, 14 7524 KALATHUR VIA, JAYAKONDAM TK- ARIYALUR-0 PANNEERSELVAM.S, S/ O SUBRAMANIAN, PUTHUKUDI PO, 15 7525 VARIYANKAVAL VIA, UDAYARPALAYAM TK- ARIYALUR-621806 P.VELMURUGAN, S/O.P ANDURENGAN,, MULLURAKUNY VGE,, 16 7526 ADAMKURICHY PO,, ARIYALUR-0 Page 3 of 108 CANDIDATE NAME SL.NO APP.NO AND ADDRESS B.KALIYAPERUMAL, S/ O.T.S.BOORASAMY,, THANDALAI NORTH ST, 17 7527 KALLATHUR THANDALAI PO, UDAYARPALAYAM TK.- ARIYALUR L.LAKSHMIKANTHAN, S/O.M.LAKSHMANAN, SOUTH STREET,, 18 7528 THIRUKKANOOR PATTI, THANJAVUR-613303 SIVAKUMAR K.S., S/O.SEPPERUMAL.E. -

List of the Candidates Short Listed for the Post of Office Assistant to Attend the Interview at Principal District Court, Ariyalur
LIST OF THE CANDIDATES SHORT LISTED FOR THE POST OF OFFICE ASSISTANT TO ATTEND THE INTERVIEW AT PRINCIPAL DISTRICT COURT, ARIYALUR Scheduled Caste - General - Non Priority Turn 52 Sl. Application No. Name and Address Date & Time No. Samathanam.V, s/o Vaiyapuri, Cholankudikadu, 26.03.2017 1 Employment Manappathur post, 09.00 A.M Sendurai Taluk, Ariyalur. Manimaran.A, s/o Athimoolam, 26.03.2017 2 Employment K.K.Nagar, 09.00 A.M Sendurai post & Taluk, Ariyalur Kaliyaperumal.R, s/o Ramalingam, Muthunayakan kuppam, 26.03.2017 3 Employment Kovilvalkai post, 09.00 A.M Udayarpalayam, Ariyalur. Kudiyarasu.K, 1-127 Athanakurichi, Mudukulam, 26.03.2017 4 Employment Athanakurichi, 09.00 A.M Sendurai, Ariyalur. Nallamuthu.T, s/o Thangavel, 26.03.2017 5 Employment Unjini post, 09.00 A.M Sendurai, Ariyalur. K.Rajendran, Azhagapuram Village & Post, 26.03.2017 6 Ex-Servicemen Udayarpalayam Taluk, 09.00 A.M Ariyalur District – 608901. Yogeswaran.K, s/o Kuganathan, 26.03.2017 7 OA – 1 No.49, Periya theru, 09.00 A.M Ariyalur Taluk & District. Mohanraj.M, s/o Mari, 26.03.2017 8 OA – 6 old No.1/21, New No.1/56, 09.00 A.M Colony street, Sundakudi, Ariyalur. Rajesh.P, s/o Panneerselvam, 26.03.2017 9 OA – 9 20, Eatutheru, Manaparai taluk, 09.00 A.M Trichy. Prabakaran.M, s/o Maruthamuthu, 26.03.2017 10 OA – 11 2, Koiltheru, Keelakangiyanur, 09.00 A.M Alanthuraiyarkattalai, Sundakudi, Ariyalur. Senthil.P, s/o Panchanathan, 26.03.2017 11 OA – 16 periyakarukai, 09.00 A.M Nagamanthal post, Jayankondam, Ariyalur. -
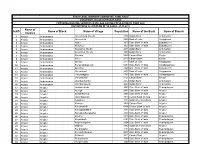
S.NO Name of District Name of Block Name of Village Population Name
STATE LEVEL BANKERS' COMMITTEE, TAMIL NADU CONVENOR: INDIAN OVERSEAS BANK PROVIDING BANKING SERVICES IN VILLAGE HAVING POPULATION OF OVER 2000 DISTRICTWISE ALLOCATION OF VILLAGES -01.11.2011 Name of S.NO Name of Block Name of Village Population Name of the Bank Name of Branch District 1 Ariyalur Andiamadam Anikudichan (South) 2730 Indian Bank Andimadam 2 Ariyalur Andiamadam Athukurichi 5540 Bank of India Alagapuram 3 Ariyalur Andiamadam Ayyur 3619 State Bank of India Edayakurichi 4 Ariyalur Andiamadam Kodukkur 3023 State Bank of India Edayakurichi 5 Ariyalur Andiamadam Koovathur (North) 2491 Indian Bank Andimadam 6 Ariyalur Andiamadam Koovathur (South) 3909 Indian Bank Andimadam 7 Ariyalur Andiamadam Marudur 5520 Canara Bank Elaiyur 8 Ariyalur Andiamadam Melur 2318 Canara Bank Elaiyur 9 Ariyalur Andiamadam Olaiyur 2717 Bank of India Alagapuram 10 Ariyalur Andiamadam Periakrishnapuram 5053 State Bank of India Varadarajanpet 11 Ariyalur Andiamadam Silumbur 2660 State Bank of India Edayakurichi 12 Ariyalur Andiamadam Siluvaicheri 2277 Bank of India Alagapuram 13 Ariyalur Andiamadam Thirukalappur 4785 State Bank of India Varadarajanpet 14 Ariyalur Andiamadam Variyankaval 4125 Canara Bank Elaiyur 15 Ariyalur Andiamadam Vilandai (North) 2012 Indian Bank Andimadam 16 Ariyalur Andiamadam Vilandai (South) 9663 Indian Bank Andimadam 17 Ariyalur Ariyalur Andipattakadu 3083 State Bank of India Reddipalayam 18 Ariyalur Ariyalur Arungal 2868 State Bank of India Ariyalur 19 Ariyalur Ariyalur Edayathankudi 2008 State Bank of India Ariyalur 20 Ariyalur -

SO-45-2013 Ariyalur
fbr6lppnq5ron$)m Gg,frg;zu€b6o6iruru6, Gleafinnan - 600 106 TAMILNADU STATE ELECTION CCMMISSION, CHENNAI_ 600 106. arlr ltnot s6f)ft'dr STATUTORYORDER eq5sso gfluq ABSTRACT ELECTIONS- OrdinaryElections to Rural Local Bodies- October 20L1 - Ariyalur District- ContestedCandidates - Accountsof electionexpenses - Failureto lodge - Show cause notices issued Failed to submit explanation and accounts Disqualification- Ordered. S.O. No. 45 / 20L3/TNSEC/ PE-2 Dated,the 21't October,2013 Read: 1, S,O.No.39/201I/TNSEC/EE, dated, the 15thSeptember 2OLL 2. S.O.No.38/2OLt/TNSEC/EE, dated, the 15'nSeptember 2OLL 3. Fromthe AriyalurDistrict Election Officer/District Collector, Lr.No.wet4/2184/24L2, dated 19. 1 1. 2012. 4. Show cause notice issued as per the Tamil Nadu State Election CommissionLr.No.6153/20t2/PEZ, dated 5.72.20L2. 5. Fromthe AriyalurDistrict Election Officer/District Collector, Lr.No.sa{.afi/4826/2072, dated 13.3.20L3. 6. Tamil Nadu State ElectionCommission letter No: 6L53/2012/PE2, dated28.5.2013. 7. Fromthe AriyalurDistrict Election Officer/District Collector, Lr.No. wafi/4826/2012,dated 24.6.20L3. 8. Tamil Nadu State ElectionCommission letter No: 6153/2AL2/PE2, dated19.9.2013. 9. Fromthe AriyalurDistrict Election Officer/District Collector, Lr.No.ooou8/4826 /20L2, dated 25.9.2013. 10.TamilNadu State ElectionCommission letter No: 6L53/20L2/PE2, dated18.10.2013. 11. Fromthe AriyalurDistrict Election Officer/District Collector, Lr.No.wa8/4826/20t2, dated 18.10.2013. ORDER: WHEREAS,in the Notiflcationissued with the S.O.first read above, by invoking sub-rule(3) of the rule 120of the TamilNadu Panchayats (Elections) Rules, 1995, the Comr-nissiondirected that all the contestingcandidates in the electionslisted therein shalllodge a true copy of their electionexpenses kept by them or by their respective electionagent under sub-rule(1) of rule L20 of the said Rules in the format prescribedtherefor under sub-rule(2) of the rule 120 of the said Ruleswith the officersmentioned therein, within thirty days from the date of declarationof the resultof the elections; z 2. -

Ariyalur District
CENSUS OF INDIA 2011 TOTAL POPULATION AND POPULATION OF SCHEDULED CASTES AND SCHEDULED TRIBES FOR VILLAGE PANCHAYATS AND PANCHAYAT UNIONS ARIYALUR DISTRICT DIRECTORATE OF CENSUS OPERATIONS TAMILNADU ABSTRACT ARIYALUR DISTRICT No. of Total Total Sl. No. Panchayat Union Total Male Total SC SC Male SC Female Total ST ST Male ST Female Village Population Female 1 Ariyalur 37 1,10,558 55,660 54,898 28,210 14,361 13,849 960 468 492 2 Thirumanur 36 1,16,349 57,007 59,342 23,979 11,678 12,301 249 126 123 3 Jayamkondam 35 1,17,515 58,360 59,155 32,388 16,099 16,289 2,906 1,441 1,465 4 Andimadam 30 1,10,115 54,791 55,324 21,811 10,837 10,974 2,339 1,172 1,167 5 T. Palur 33 1,06,142 52,799 53,343 24,772 12,435 12,337 2,148 1,045 1,103 6 Sendurai 30 1,10,421 54,636 55,785 32,610 16,447 16,163 1,344 645 699 Grand Total 201 6,71,100 3,33,253 3,37,847 1,63,770 81,857 81,913 9,946 4,897 5,049 ARIYALUR PANCHAYAT UNION Sl. Name of Population Scheduled Castes Scheduled Tribes No. Panchayat Total Males Females Total Males Females Total Males Females 1 2 3 4 5 6 7 8 9 10 11 1 Alanduraiyarkattalai 2,106 1,090 1,016 427 220 207 - - - 2 Andipattakkadu 1,963 1,017 946 372 205 167 - - - 3 Arungal 3,581 1,767 1,814 827 415 412 259 120 139 4 Edayathankudi 2,191 1,135 1,056 109 52 57 81 43 38 5 Eruthukaranpatti 4,801 2,419 2,382 531 266 265 246 127 119 6 Govindapuram 1,468 759 709 363 190 173 - - - 7 Hasthinapuram 1,854 931 923 428 215 213 - - - 8 Iluppaiyur 4,142 2,158 1,984 1,985 1,043 942 - - - 9 Kadugur 5,662 2,890 2,772 855 437 418 1 1 - 10 Kallankurichi 1,869