A Cytological Study of the Zingiberales with Special Reference to Their Taxonomy1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ensete Ventricosum: a Multipurpose Crop Against Hunger in Ethiopia
Hindawi e Scientific World Journal Volume 2020, Article ID 6431849, 10 pages https://doi.org/10.1155/2020/6431849 Review Article Ensete ventricosum: A Multipurpose Crop against Hunger in Ethiopia Getahun Yemata Bahir Dar University, College of Science, Department of Biology, Mail-79, Bahir Dar, Ethiopia Correspondence should be addressed to Getahun Yemata; [email protected] Received 2 October 2019; Accepted 20 December 2019; Published 6 January 2020 Academic Editor: Tadashi Takamizo Copyright © 2020 Getahun Yemata. (is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Ensete ventricosum is a traditional multipurpose crop mainly used as a staple/co-staple food for over 20 million people in Ethiopia. Despite this, scientific information about the crop is scarce. (ree types of food, viz., Kocho (fermented product from scraped pseudostem and grated corm), Bulla (dehydrated juice), and Amicho (boiled corm) can be prepared from enset. (ese products are particularly rich in carbohydrates, minerals, fibres, and phenolics, but poor in proteins. Such meals are usually served with meat and cheese to supplement proteins. As a food crop, it has useful attributes such as foods can be stored for long time, grows in wide range of environments, produces high yield per unit area, and tolerates drought. It has an irreplaceable role as a feed for animals. Enset starch is found to have higher or comparable quality to potato and maize starch and widely used as a tablet binder and disintegrant and also in pharmaceutical gelling, drug loading, and release processes. -

Promecothecini Chapuis 1875 Promecothecites Chapuis 1875:300
Tribe Promecothecini Chapuis 1875 Promecothecites Chapuis 1875:300. Handlirsch 1925:666 (classification); Gressitt 1950:81 (China species). Promecothecini Chapuis. Würmli 1975a:45 (genera); Bouchard et al. 2011:78, 518 (nomenclature); Liao et al. 2015:162 (host plants). Promecothecini Weise 1911a:78. Weise 1911b:81 (redescription); Zacher 1913:103 (key); Handlirsch 1925:666 (classification); Uhmann 1931i:848 (museum list), 1940g:121 (claws), 1951a:31 (museum list), 1958e:222 (catalog), 1959d:8 (scutellum), 1964a:458 (catalog), 1964(1965):241 (faunal list), 1966d:275 (note); Bryant 1936:256 (faunal list); Liu 1936:249 (China species); Wu 1937:912 (faunal list); Gressitt 1939c:133 (distribution), 1957b:279 (South Pacific species), 1970:71 (Fiji species); Gressitt & Kimoto 1963a:905 (China species); Seeno & Wilcox 1982:164 (catalog); Jolivet 1988b:13 (host plants), 1989b:310 (host plants); Jolivet & Hawkeswood 1995:154 (host plants); Cox 1996a:172 (pupae); Mohamedsaid 2004:169 (Malaysian species); Staines 2004a:317 (host plants); Chaboo 2007:183 (phylogeny). Type genus:Promecotheca Blanchard. Promecispa Weise 1909 Promecispa Weise 1909:112. Type species:Promecispa voeltzkowi Weise 1909 by monotypy. Weise 1910d:442, 501 (faunal list), 1911a:53 (catalog), 1911b:80 (redescription); Uhmann 1931i:848 (museum list), 1958e:223 (catalog); Würmli 1975a:46 (genera); Seeno & Wilcox 1982:164 (catalog). Promecispa voeltzkowi Weise 1909 Promecispa voeltzkowi Weise 1909:112 (type:Madagascar, Kinkuni, ZMHB). Weise 1910d:442, 501 (faunal list), 1911a:53 (catalog), 1911b:80 (catalog); Uhmann 1931i:848 (type), 1958e:223 (catalog). Distribution. Madagascar. Food plant. Unknown. Promecotheca Blanchard 1853 Promecotheca Dejean 1837:387 Nomen Nudum. Guérin-Méneville 1840b:334 (note). Promecotheca Blanchard 1853:312. Type species:Hispa cyanipes Erichson 1834, designated by Baly 1858. -

Well-Known Plants in Each Angiosperm Order
Well-known plants in each angiosperm order This list is generally from least evolved (most ancient) to most evolved (most modern). (I’m not sure if this applies for Eudicots; I’m listing them in the same order as APG II.) The first few plants are mostly primitive pond and aquarium plants. Next is Illicium (anise tree) from Austrobaileyales, then the magnoliids (Canellales thru Piperales), then monocots (Acorales through Zingiberales), and finally eudicots (Buxales through Dipsacales). The plants before the eudicots in this list are considered basal angiosperms. This list focuses only on angiosperms and does not look at earlier plants such as mosses, ferns, and conifers. Basal angiosperms – mostly aquatic plants Unplaced in order, placed in Amborellaceae family • Amborella trichopoda – one of the most ancient flowering plants Unplaced in order, placed in Nymphaeaceae family • Water lily • Cabomba (fanwort) • Brasenia (watershield) Ceratophyllales • Hornwort Austrobaileyales • Illicium (anise tree, star anise) Basal angiosperms - magnoliids Canellales • Drimys (winter's bark) • Tasmanian pepper Laurales • Bay laurel • Cinnamon • Avocado • Sassafras • Camphor tree • Calycanthus (sweetshrub, spicebush) • Lindera (spicebush, Benjamin bush) Magnoliales • Custard-apple • Pawpaw • guanábana (soursop) • Sugar-apple or sweetsop • Cherimoya • Magnolia • Tuliptree • Michelia • Nutmeg • Clove Piperales • Black pepper • Kava • Lizard’s tail • Aristolochia (birthwort, pipevine, Dutchman's pipe) • Asarum (wild ginger) Basal angiosperms - monocots Acorales -

Alpinia Galanga (L.) Willd
TAXON: Alpinia galanga (L.) Willd. SCORE: 5.0 RATING: Low Risk Taxon: Alpinia galanga (L.) Willd. Family: Zingiberaceae Common Name(s): false galangal Synonym(s): Languas galanga (L.) Stuntz greater galanga Maranta galanga L. languas Siamese-ginger Thai ginger Assessor: Chuck Chimera Status: Assessor Approved End Date: 16 Jun 2016 WRA Score: 5.0 Designation: L Rating: Low Risk Keywords: Rhizomatous, Naturalized, Edible, Self-Compatible, Pollinator-Limited Qsn # Question Answer Option Answer 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? 103 Does the species have weedy races? Species suited to tropical or subtropical climate(s) - If 201 island is primarily wet habitat, then substitute "wet (0-low; 1-intermediate; 2-high) (See Appendix 2) High tropical" for "tropical or subtropical" 202 Quality of climate match data (0-low; 1-intermediate; 2-high) (See Appendix 2) Low 203 Broad climate suitability (environmental versatility) y=1, n=0 y Native or naturalized in regions with tropical or 204 y=1, n=0 y subtropical climates Does the species have a history of repeated introductions 205 y=-2, ?=-1, n=0 y outside its natural range? 301 Naturalized beyond native range y = 1*multiplier (see Appendix 2), n= question 205 y 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see Appendix 2) n 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) n 304 Environmental weed n=0, y = 2*multiplier (see Appendix 2) n 305 Congeneric weed n=0, y = 1*multiplier (see Appendix 2) y 401 Produces spines, thorns or burrs y=1, n=0 n 402 Allelopathic 403 Parasitic y=1, n=0 n 404 Unpalatable to grazing animals 405 Toxic to animals y=1, n=0 n 406 Host for recognized pests and pathogens y=1, n=0 n 407 Causes allergies or is otherwise toxic to humans y=1, n=0 n Creation Date: 16 Jun 2016 (Alpinia galanga (L.) Willd.) Page 1 of 15 TAXON: Alpinia galanga (L.) Willd. -
![Ensete Ventricosum (Welw.) Cheesman]](https://docslib.b-cdn.net/cover/5290/ensete-ventricosum-welw-cheesman-185290.webp)
Ensete Ventricosum (Welw.) Cheesman]
73 Fruits (6), 342–348 | ISSN 0248-1294 print, 1625-967X online | https://doi.org/10.17660/th2018/73.6.4 | © ISHS 2018 Review article – Thematic Issue Traditional enset [Ensete ventricosum (Welw.) Cheesman] improvement sucker propagation methods and opportunities for crop Z. Yemataw , K. Tawle 3 1 1 2,a 1 , G. Blomme and K. Jacobsen 23 The Southern Agricultural Research Institute (SARI-Areka), Areka Agricultural Research Center, P.O. Box 79, Areka, Ethiopia Bioversity International, c/o ILRI, P.O. Box 5689, Addis Ababa, Ethiopia Royal Museum for Central Africa, Leuvensesteenweg 13, 3080 Tervuren, Belgium Summary Significance of this study Introduction – This review focuses on the enset What is already known on this subject? seed systems in Ethiopia and explores opportunities • to improve the system. Cultivated enset is predomi- nantly vegetatively propagated by farmers. Repro- Traditional macro-propagation methods, using entire duction of an enset plant from seed is seldom prac- scaperhizomes level. or rhizome pieces, currently suffice to pro- ticed by farmers and has been reported only from vide the needed enset suckers at farm, village or land- the highlands of Gardula. Seedlings arising from seed What are the new findings? are reported to be less vigorous than the suckers • e.g., obtained through vegetative propagation. Rhizomes when introducing a new enset cultivar or coping with from immature plants, between 2 and 6 years old, severeWhen larger disease quantities or pest impacts, of suckers improved/novel are needed, mi are preferred for the production of suckers. The aver- age number of suckers produced per rhizome ranges this review paper, could offer solutions. -

Strelitzia Nicolai (Strelitziaceae): a New Species, Genus and Family Weed Record for New South Wales
Volume 20: 1–3 ELOPEA Publication date: 30 January 2017 T dx.doi.org/10.7751/telopea11022 Journal of Plant Systematics plantnet.rbgsyd.nsw.gov.au/Telopea • escholarship.usyd.edu.au/journals/index.php/TEL • ISSN 0312-9764 (Print) • ISSN 2200-4025 (Online) Strelitzia nicolai (Strelitziaceae): a new species, genus and family weed record for New South Wales Marco F Duretto1,4, Seanna McCune1, Reece Luxton2 and Dennis Milne3 1National Herbarium of New South Wales, Royal Botanic Gardens & Domain Trust, Mrs Macquaries Road, Sydney, NSW 2000, Australia. 2Clarence Valley Council, Locked Bag 23, Grafton, NSW 2460, Australia. 3Yuraygir Landcare, Minnie Water, NSW 2462, Australia. 4Author for correspondence: [email protected] Abstract Strelitzia nicolai Regel & Körn. (Strelitziaceae), a native of South Africa, is newly recorded as a sparingly naturalised weed for New South Wales and represents new family, generic and species records for the state. Descriptions, notes and identification key are provided for the family, genus and species. Introduction Strelitzia nicolai Regel & Körn. (Giant White Bird of Paradise or Natal Wild Banana; Strelitziaceae), a native of South Africa, is a common horticultural subject in eastern Australia. Recently a small colony of plants was discovered at Minnie Water (c. 60 km NNE of Coffs Harbour, North Coast, New South Wales). The colony is of note as some plants were 8 m tall (suggesting they had been there for some time) and that they were setting viable seed. Seedlings were found within this population and Milne and Luxton have observed that the species is being found in increasing numbers on council land and in National Parks of the area. -

ORNAMENTAL GARDEN PLANTS of the GUIANAS: an Historical Perspective of Selected Garden Plants from Guyana, Surinam and French Guiana
f ORNAMENTAL GARDEN PLANTS OF THE GUIANAS: An Historical Perspective of Selected Garden Plants from Guyana, Surinam and French Guiana Vf•-L - - •• -> 3H. .. h’ - — - ' - - V ' " " - 1« 7-. .. -JZ = IS^ X : TST~ .isf *“**2-rt * * , ' . / * 1 f f r m f l r l. Robert A. DeFilipps D e p a r t m e n t o f B o t a n y Smithsonian Institution, Washington, D.C. \ 1 9 9 2 ORNAMENTAL GARDEN PLANTS OF THE GUIANAS Table of Contents I. Map of the Guianas II. Introduction 1 III. Basic Bibliography 14 IV. Acknowledgements 17 V. Maps of Guyana, Surinam and French Guiana VI. Ornamental Garden Plants of the Guianas Gymnosperms 19 Dicotyledons 24 Monocotyledons 205 VII. Title Page, Maps and Plates Credits 319 VIII. Illustration Credits 321 IX. Common Names Index 345 X. Scientific Names Index 353 XI. Endpiece ORNAMENTAL GARDEN PLANTS OF THE GUIANAS Introduction I. Historical Setting of the Guianan Plant Heritage The Guianas are embedded high in the green shoulder of northern South America, an area once known as the "Wild Coast". They are the only non-Latin American countries in South America, and are situated just north of the Equator in a configuration with the Amazon River of Brazil to the south and the Orinoco River of Venezuela to the west. The three Guianas comprise, from west to east, the countries of Guyana (area: 83,000 square miles; capital: Georgetown), Surinam (area: 63, 037 square miles; capital: Paramaribo) and French Guiana (area: 34, 740 square miles; capital: Cayenne). Perhaps the earliest physical contact between Europeans and the present-day Guianas occurred in 1500 when the Spanish navigator Vincente Yanez Pinzon, after discovering the Amazon River, sailed northwest and entered the Oyapock River, which is now the eastern boundary of French Guiana. -

C-23 Phytochemical of Kaempferia Plant And
Proceeding of International Conference On Research, Implementation And Education Of Mathematics And Sciences 2014, Yogyakarta State University, 18-20 May 2014 C-23 PHYTOCHEMICAL OF KAEMPFERIA PLANT AND BIOPROSPECTING FOR CANCER TREATMENT Sri Atun Chemistry education Faculty of Mathematical and Natural Science, Yogyakarta State University, Jl. Colombo No. 1 Yogyakarta, Indonesia, 55281 e-mail : [email protected] ABSTRACT Kaempferia genus is perennial member of the Zingiberaceae family and is cultivated in Indonesia and other parts of Southeast Asia. Number of studies has been conducted, providing information related to Kaempferia as antioxidant; antimutagenic; and chemopreventive agent. This paper reports some isolated compounds from this plant, biological activity, and bioprospecting for cancer treatment. Keyword: Cancer treatment; Kaempferia; Zingiberaceae INTRODUCTION Kaempferia is a genus, belong to family of Zingiberaceae. This plant grows in Southeast Asia, India, Sri Lanka, Indonesia, and Southem China. Kaempferia genus sinonim with Boesenbergia genus by Baker. This plant has 8 different botanical names which are Boesenbergia cochinchinensis (Gagnep.) Loes., Boesenbergia pandurata (Roxb.) Schltr., Curcuma rotunda L., Gastrochilus panduratus (Roxb.) Ridl., Gastrochilus rotundus (L.) Alston, Kaempferia cochinchinensis Gagnep., Kaempferia ovate Roscoe, Kaempferia galanga, Kaempferia rotunda, and Kaempferia pandurata Roxb nonetheless it is currently known as Boesenbergia rotunda (L.)Mansf (Tan Eng-Chong, et. al, 2012). The plants grown naturally in damp, shaded parts of the lowland or on hill slopes, as scattered plants or thickets. Economically important species among the plant families, the Zingiberaceae, which are perennial rhizomatous herbs, contain volatile oil and other important compounds of enormous medicinal values (Singh C.B., 2013). Phytochemical and biologycal activities of some species of Kaempferia Phytochemical and biologycal some species of plants of the genus Kaempferia reported by many researchers, among others: 1. -

Seed Germination and Genetic Structure of Two Salvia Species In
Seed germination and genetic structure of two Salvia species in response to environmental variables among phytogeographic regions in Jordan (Part I) and Phylogeny of the pan-tropical family Marantaceae (Part II). Dissertation Zur Erlangung des akademischen Grades Doctor rerum naturalium (Dr. rer. nat) Vorgelegt der Naturwissenschaftlichen Fakultät I Biowissenschaften der Martin-Luther-Universität Halle-Wittenberg Von Herrn Mohammad Mufleh Al-Gharaibeh Geb. am: 18.08.1979 in: Irbid-Jordan Gutachter/in 1. Prof. Dr. Isabell Hensen 2. Prof. Dr. Martin Roeser 3. Prof. Dr. Regina Classen-Bockhof Halle (Saale), den 10.01.2017 Copyright notice Chapters 2 to 4 have been either published in or submitted to international journals or are in preparation for publication. Copyrights are with the authors. Just the publishers and authors have the right for publishing and using the presented material. Therefore, reprint of the presented material requires the publishers’ and authors’ permissions. “Four years ago I started this project as a PhD project, but it turned out to be a long battle to achieve victory and dreams. This dissertation is the culmination of this long process, where the definition of “Weekend” has been deleted from my dictionary. It cannot express the long days spent in analyzing sequences and data, battling shoulder to shoulder with my ex- computer (RIP), R-studio, BioEdite and Microsoft Words, the joy for the synthesis, the hope for good results and the sadness and tiredness with each attempt to add more taxa and analyses.” “At the end, no phrase can describe my happiness when I saw the whole dissertation is printed out.” CONTENTS | 4 Table of Contents Summary .......................................................................................................................................... -
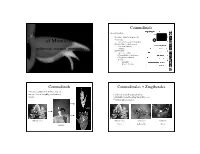
Diversity and Evolution of Monocots
Commelinids 4 main groups: Diversity and Evolution • Acorales - sister to all monocots • Alismatids of Monocots – inc. Aroids - jack in the pulpit • Lilioids (lilies, orchids, yams) – non-monophyletic . spiderworts, bananas, pineapples . – petaloid • Commelinids – Arecales – palms – Commelinales – spiderwort – Zingiberales –banana – Poales – pineapple – grasses & sedges Commelinids Commelinales + Zingiberales • theme: reduction of flower, loss of nectar, loss of zoophily, evolution of • 2 closely related tropical orders bracts • primarily nectar bearing but with losses • bracted inflorescences grass pickeral weed pickeral weed spiderwort heliconia nectar pollen only bracts rapatead bromeliad Commelinaceae - spiderwort Commelinaceae - spiderwort Family of small herbs with succulent stems, stems jointed; leaves sheathing. Family does not produce Inflorescence often bracted nectar, but showy flowers for insect pollen gathering. Rhoeo - Moses in a cradle Commelina erecta - Erect dayflower Tradescantia ohiensis - spiderwort Tradescantia ohiensis - spiderwort Commelinaceae - spiderwort Commelinaceae - spiderwort Flowers actinomorphic or • species rich in pantropics, CA 3 CO 3 A 6 G (3) zygomorphic especially Africa • floral diversity is enormous Commelina communis - day flower Tradescantia ohiensis - spiderwort Pontederiaceae - pickerel weed Pontederiaceae - pickerel weed Aquatic family of emergents or floaters. Pickerel weed has glossy heart-shaped leaves, Water hyacinth (Eichhornia) from superficially like Sagittaria but without net venation. -

Thai Zingiberaceae : Species Diversity and Their Uses
URL: http://www.iupac.org/symposia/proceedings/phuket97/sirirugsa.html © 1999 IUPAC Thai Zingiberaceae : Species Diversity And Their Uses Puangpen Sirirugsa Department of Biology, Faculty of Science, Prince of Songkla University, Hat Yai, Thailand Abstract: Zingiberaceae is one of the largest families of the plant kingdom. It is important natural resources that provide many useful products for food, spices, medicines, dyes, perfume and aesthetics to man. Zingiber officinale, for example, has been used for many years as spices and in traditional forms of medicine to treat a variety of diseases. Recently, scientific study has sought to reveal the bioactive compounds of the rhizome. It has been found to be effective in the treatment of thrombosis, sea sickness, migraine and rheumatism. GENERAL CHARACTERISTICS OF THE FAMILY ZINGIBERACEAE Perennial rhizomatous herbs. Leaves simple, distichous. Inflorescence terminal on the leafy shoot or on the lateral shoot. Flower delicate, ephemeral and highly modified. All parts of the plant aromatic. Fruit a capsule. HABITATS Species of the Zingiberaceae are the ground plants of the tropical forests. They mostly grow in damp and humid shady places. They are also found infrequently in secondary forest. Some species can fully expose to the sun, and grow on high elevation. DISTRIBUTION Zingiberaceae are distributed mostly in tropical and subtropical areas. The center of distribution is in SE Asia. The greatest concentration of genera and species is in the Malesian region (Indonesia, Malaysia, Singapore, Brunei, the Philippines and Papua New Guinea) *Invited lecture presented at the International Conference on Biodiversity and Bioresources: Conservation and Utilization, 23–27 November 1997, Phuket, Thailand. -

Pollination and Botanic Gardens Contribute to the Next Issue of Roots
Botanic Gardens Conservation International Education Review Volume 17 • Number 1 • May 2020 Pollination and botanic gardens Contribute to the next issue of Roots The next issue of Roots is all about education and technology. As this issue goes to press, most botanic gardens around the world are being impacted by the spread of the coronavirus Covid-19. With many Botanic Gardens Conservation International Education Review Volume 16 • Number 2 • October 2019 Citizen gardens closed to the public, and remote working being required, Science educators are having to find new and innovative ways of connecting with visitors. Technology is playing an ever increasing role in the way that we develop and deliver education within botanic gardens, making this an important time to share new ideas and tools with the community. Have you developed a new and innovative way of engaging your visitors through technology? Are you using technology to engage a Botanic Gardens Conservation International Education Review Volume 17 • Number 1 • April 2020 wider audience with the work of your garden? We are currently looking for a variety of contributions including Pollination articles, education resources and a profile of an inspirational garden and botanic staff member. gardens To contribute, please send a 100 word abstract to [email protected] by 15th June 2020. Due to the global impacts of COVID-19, BGCI’s 7th Global Botanic Gardens Congress is being moved to the Australian spring. Join us in Melbourne, 27 September to 1 October 2021, the perfect time to visit Victoria. Influence and Action: Botanic Gardens as Agents of Change will explore how botanic gardens can play a greater role in shaping our future.