Corneal Topography
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Surgically Induced Scleral Staphyloma
Original Article Surgically induced scleral staphyloma Yong Yao1, Ming-Zhi Zhang1, Vishal Jhanji1,2 1Joint International Eye Center of Shantou University and The Chinese University of Hong Kong, Shantou 515041, China; 2Department of Ophthalmology and Visual Sciences, The Chinese University of Hong Kong, Hong Kong, China Contributions: (I) Conception and design: Y Yao; (II) Administrative support: MZ Zhang; (III) Provision of study materials or patients: Y Yao; (IV) Collection and assembly of data: Y Yao; (V) Data analysis and interpretation: Y Yao; V Jhanji; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Yong Yao. Shantou International Eye Center, Jinping District, Guangxia New Town, Shantou 515041, China. Email: [email protected] or [email protected]. Background: To report the clinical features of surgically induced scleral staphyloma and investigate the management. Methods: Retrospective uncontrolled study. Results: A full ophthalmological evaluation of surgically induced scleral staphyloma in four patients was performed. The first patient was a 3-year-old young girl underwent corneal dermoid resection. The second patient was a 60-year-old man underwent nasal pterygium excision and conjunctival autograft without Mitomycin C (MMC). The other two were respectively a 74-year-old woman and a 69-year-old man underwent cataract surgery. All patients performed allogeneic sclera patch graft. In the at least half a year follow-up, the best corrected visual acuity (BCVA) of all the four patients were no worse than that of preoperative. Ocular symptoms disappeared, including eye pain, foreign body sensation, and so on. Unfortunately, the fourth patient showed sclera rejection and partial dissolution at postoperative 1 month. -

Long-Term Ocular Surface Stability in Conjunctival Limbal Autograft Donor Eyes
CLINICAL SCIENCE Long-Term Ocular Surface Stability in Conjunctival Limbal Autograft Donor Eyes Albert Y. Cheung, MD,*† Enrica Sarnicola, MD,*†‡ and Edward J. Holland, MD*† (OSST) procedures can provide healthy limbal stem cells Purpose: fi – To investigate the incidence of limbal stem cell de ciency and conjunctiva to rehabilitate a damaged ocular surface.2 8 (LSCD) in donor eyes after conjunctival limbal autograft (CLAU). Conjunctival limbal autograft (CLAU) is a technique that ; Methods: An observational retrospective review was performed on transplants a portion (typically 3to6clockhours)ofthe all patients who underwent CLAU alone, combined keratolimbal healthy stem cells from an unaffected eye to the contralat- allograft with CLAU (“Modified Cincinnati Procedure”), or com- eral affected eye in individuals affected by unilateral LSCD.6 bined living-related conjunctival limbal allograft (lr-CLAL) with 7 $ Cultivated limbal epithelial transplantation (CLET) CLAU having 6 months of follow-up after surgery. The outcome 8 measures were best-corrected visual acuity (BCVA) and ocular and simple limbal epithelial transplantation (SLET) provide surface status. ex vivo and in vivo expansion of stem cells, respectively, to repopulate the ocular surface. In contrast to CLET and SLET, Results: The inclusion criteria were fulfilled by 45 patients. Of CLAU is ideal for rehabilitating severe unilateral LSCD these, 26 patients underwent CLAU, 18 underwent combined requiring combined conjunctival limbal transplantation and keratolimbal allograft/CLAU, and 1 underwent combined lr- for reconstruction of the ocular surface. Proponents of CLET CLAL/CLAU. Mean age at the time of surgery was 39.6 years. and SLET have raised concerns regarding the potential Mean logMAR preoperative BCVA was 20.08. -

Managing Ocular Surface Inflammation”
E Y 7th EDITION OLOGIES OF THE E OLOGIES OF th H 7 AT EDITION P THEA INTERNATIONAL CONTEST OF CLINICAL CASES IN PATHOLOGIES OF THE EYE THEA INTERNATIONAL CONTEST OF CLINICAL CASES IN PATHOLOGIES OF THE EYE NTEST OF CLINICAL CASES IN OF CLINICAL NTEST O This brochure is produced under the sole responsibility of the authors. Laboratoires Théa are not involved in the writing of this document. This brochure may contain MA off-label and/or not validated by health authorities information. NATIONAL C NATIONAL R HEA INTE HEA T 2018 - 2019 EDITION 2018 - 2019 EDITION MANAGING MANAGING OCULAR SURFACE OCULAR SURFACE INFLAMMATION THE BEST CLINICAL CASES FROM 22 COUNTRIES INFLAMMATION THE BEST CLINICAL CASES FROM 22 COUNTRIES WWW.THEA-TROPHY.COM CLINICAL CASES CLINICAL - BA WTROBRO0120 WWW.THEA-TROPHY.COM Laboratoires Théa • 12, rue Louis Blériot 63017 Clermont-Ferrand Cedex 2 • France T +33 4 73 98 14 36 Laboratoires Théa F +33 4 73 98 14 38 LABORATOIRES-THEA.COM th 7 EDITION This brochure is produced under the sole responsibility of the authors, Laboratoires Théa are not involved in the writing of the clinical cases. This brochure may contain MA off-label and/or not validated by health authorities information. PREFACE Mr. JEAN-FRÉDÉRIC CHIBRET TROPHY 2018-2019 the Clinical Cases 2 M. Jean-Frédéric CHIBRET President of Laboratoires Théa Théa has focused its activities on research, development and commercialization of eye-care products, but at the same time has invested in education and knowledge promotion in line with the strong Chibret family tradition. In this way, Théa supports many projects and educational activities such as the European Meeting of Young Ophthalmologists (EMYO) or its partnership with the European Board of Ophthalmology (EBO). -

Amniotic Membrane Grafts in Pediatric Corneal Limbal Dermoids with Conjunctival Eyelashes: a Case Presentation
Open Journal of Ophthalmology, 2016, 6, 191-197 http://www.scirp.org/journal/ojoph ISSN Online: 2165-7416 ISSN Print: 2165-7408 Amniotic Membrane Grafts in Pediatric Corneal Limbal Dermoids with Conjunctival Eyelashes: A Case Presentation Larisa Akah Che1, Linda A. Morgan2, Donny W. Suh2,3 1University of Nebraska Medical Center, Omaha, NE, USA 2Children’s Hospital & Medical Center, Omaha, NE, USA 3Department of Ophthalmology and Visual Sciences, Truhlsen Eye Institute, University of Nebraska Medical Center, Omaha, NE, USA How to cite this paper: Che, L.A., Morgan, Abstract L.A. and Suh, D.W. (2016) Amniotic Mem- brane Grafts in Pediatric Corneal Limbal Purpose: To report a case of a pediatric corneal limbal dermoid with eyelashes and Dermoids with Conjunctival Eyelashes: A to describe post-operative changes after excision with reconstruction using amniotic Case Presentation. Open Journal of Oph- membrane grafting, sutures and fibrinogen-thrombin glue. Case Report: One pedia- thalmology, 6, 191-197. http://dx.doi.org/10.4236/ojoph.2016.64027 tric patient was identified with a grade II infratemporal corneal-limbal dermoid with conjunctival eyelashes. The dermoid was surgically excised and the cornea recon- Received: August 23, 2016 structed with amniotic membrane using sutures and fibrinogen/thrombin glue. Pre- Accepted: October 11, 2016 operative and postoperative measurement of astigmatism, anisometropia and pres- Published: October 14, 2016 ence of exposure keratopathy were performed. Copyright © 2016 by authors and Scientific Research Publishing Inc. Keywords This work is licensed under the Creative Commons Attribution International Corneal Limbal Dermoid, Amniotic Membrane Graft, Choriostoma License (CC BY 4.0). http://creativecommons.org/licenses/by/4.0/ Open Access 1. -

Transglutaminase in Ocular Health and Pathological Processes
perim Ex en l & ta a l ic O p in l h t C h f Journal of Clinical & Experimental a Tong et al. J Clinic Experiment Ophthalmol 2011, S:2 o l m l a o n l DOI: 10.4172/2155-9570.S2-002 r o g u y o J Ophthalmology ISSN: 2155-9570 ResearchResearch Article Article OpenOpen Access Access Recent Advances: Transglutaminase in Ocular Health and Pathological Processes Louis Tong1,2,3*, Evelyn Png2, Wanwen Lan2 and Andrea Petznick2 1Singapore National Eye Center, Singapore 2Singapore Eye Research Institute, Singapore 3Duke-NUS Graduate Medical School, Singapore Abstract Transglutaminase (TG) is a diverse class of crosslinking enzymes involved in the regulation of cytokine production, endocytosis, cell adhesion, migration, apoptosis and autophagy. It has been implicated in inflammatory diseases, neurodegenerative processes and cancer. The eye is a specialized organ which subserves the important function of vision and has distinctive physiological and anatomical properties that differ from other body tissues. Understanding of the roles of various TGs in the eye therefore require studies specific to cells and tissues of ocular origin. We review the advances in TG research in ocular diseases, including pterygium, glaucoma, cataract and proliferative vitreoretinopathy. TG1 is a molecule important for keratinisation in many cicatrizing diseases of the ocular surface, including keratoconjunctivitis sicca. TG2, with multiple functions, has been shown to be important in inflammation and cell adhesion in various ocular diseases. The results of TG research in each region of the eye are critically assessed and the implications of these studies in the treatment of ocular diseases are discussed. -

LCD): Computerized Corneal Topography (L33810
Local Coverage Determination (LCD): Computerized Corneal Topography (L33810) Links in PDF documents are not guaranteed to work. To follow a web link, please use the MCD Website. Contractor Information CONTRACTOR NAME CONTRACT TYPE CONTRACT NUMBER JURISDICTION STATE(S) First Coast Service Options, Inc. A and B MAC 09102 - MAC B J - N Florida First Coast Service Options, Inc. A and B MAC 09202 - MAC B J - N Puerto Rico First Coast Service Options, Inc. A and B MAC 09302 - MAC B J - N Virgin Islands LCD Information Document Information LCD ID Original Effective Date L33810 For services performed on or after 10/01/2015 LCD Title Revision Effective Date Computerized Corneal Topography For services performed on or after 01/08/2019 Proposed LCD in Comment Period Revision Ending Date N/A N/A Source Proposed LCD Retirement Date N/A N/A AMA CPT / ADA CDT / AHA NUBC Copyright Notice Period Start Date Statement N/A CPT codes, descriptions and other data only are copyright 2019 American Medical Association. All Rights Notice Period End Date Reserved. Applicable FARS/HHSARS apply. N/A Current Dental Terminology © 2019 American Dental Association. All rights reserved. Copyright © 2019, the American Hospital Association, Chicago, Illinois. Reproduced with permission. No portion of the AHA copyrighted materials contained within this publication may be copied without the express written consent of the AHA. AHA copyrighted materials including the UB-04 codes and descriptions may not be removed, copied, or utilized within any Created on 01/02/2020. Page 1 of 7 software, product, service, solution or derivative work without the written consent of the AHA. -

Ethical Issues in Living-Related Corneal Tissue Transplantation
Viewpoint Ethical issues in living-related corneal J Med Ethics: first published as 10.1136/medethics-2018-105146 on 23 May 2019. Downloaded from tissue transplantation Joséphine Behaegel, 1,2 Sorcha Ní Dhubhghaill,1,2 Heather Draper3 1Department of Ophthalmology, ABSTRact injury, typically chemical burns, chronic inflamma- Antwerp University Hospital, The cornea was the first human solid tissue to be tion and certain genetic diseases, the limbal stem Edegem, Belgium 2 transplanted successfully, and is now a common cells may be lost and the cornea becomes vascula- Faculty of Medicine and 5 6 Health Sciences, Dept of procedure in ophthalmic surgery. The grafts come from rised and opaque, leading to blindness (figure 1). Ophthalmology, Visual Optics deceased donors. Corneal therapies are now being In such cases, standard corneal transplants fail and Visual Rehabilitation, developed that rely on tissue from living-related donors. because of the inability to maintain a healthy epithe- University of Antwerp, Wilrijk, This presents new ethical challenges for ophthalmic lium. Limbal stem cell transplantation is designed to Belgium 3Division of Health Sciences, surgeons, who have hitherto been somewhat insulated address this problem by replacing the damaged or Warwick Medical School, from debates in transplantation and donation ethics. lost limbal stem cells (LSC) and restoring the ocular University of Warwick, Coventry, This paper provides the first overview of the ethical surface, which in turn increases the success rates of United Kingdom considerations generated by ocular tissue donation subsequent sight-restoring corneal transplants.7 8 from living donors and suggests how these might Limbal stem cell donations only entail the removal Correspondence to be addressed in practice. -

Division of Health Care Financing & Policy SB 278 Section 16
Division of Health Care Financing & Policy SB 278 Section 16 - Physician Rates Reporting Facility & Non-Facility Rate Comparison Nevada 2016 2016 Medicaid Medicaid Medicaid Medicare Medicare vs. vs. Procedure Code & Description Rates (1) Non-Facility Facility Medicare Medicare Rates for Rates for Non-Facility Facility 10021 Fna w/o image 70.92 128.90 72.80 (57.98) (1.88) 10022 Fna w/image 65.39 148.21 68.38 (82.82) (2.99) 10030 Guide cathet fluid drainage 154.73 827.01 176.71 (672.28) (21.98) 10035 Perq dev soft tiss 1st imag 86.35 568.38 90.92 (482.03) (4.57) 10036 Perq dev soft tiss add imag 43.52 495.42 45.82 (451.90) (2.30) 10040 Acne surgery 87.47 106.03 92.10 (18.56) (4.63) 10060 Drainage of skin abscess 95.60 122.68 101.60 (27.08) (6.00) 10061 Drainage of skin abscess 178.04 215.50 188.01 (37.46) (9.97) 10080 Drainage of pilonidal cyst 103.29 188.83 107.87 (85.54) (4.58) 10081 Drainage of pilonidal cyst 172.45 281.57 177.64 (109.12) (5.19) 10120 Remove foreign body 103.22 159.56 108.73 (56.34) (5.51) 10121 Remove foreign body 185.58 287.08 194.44 (101.50) (8.86) 10140 Drainage of hematoma/fluid 118.06 170.87 124.18 (52.81) (6.12) 10160 Puncture drainage of lesion 95.62 136.49 100.72 (40.87) (5.10) 10180 Complex drainage wound 178.97 258.94 188.14 (79.97) (9.17) 11000 Debride infected skin 28.42 56.88 29.76 (28.46) (1.34) 11001 Debride infected skin add-on 14.22 22.40 14.87 (8.18) (0.65) 11004 Debride genitalia & perineum 579.35 608.28 608.28 (28.93) (28.93) 11005 Debride abdom wall 781.65 822.97 822.97 (41.32) (41.32) 11006 Debride -

Current Developments in Corneal Topography and Tomography
diagnostics Review Current Developments in Corneal Topography and Tomography Piotr Kanclerz 1,2,* , Ramin Khoramnia 3 and Xiaogang Wang 4 1 Hygeia Clinic, Department of Ophthalmologyul, Ja´skowaDolina 57, 80-286 Gda´nsk,Poland 2 Helsinki Retina Research Group, University of Helsinki, 00100 Helsinki, Finland 3 The David J. Apple International Laboratory for Ocular Pathology, Department of Ophthalmology, University of Heidelberg, 69120 Heidelberg, Germany; [email protected] 4 Department of Cataract, Shanxi Eye Hospital, Taiyuan 030002, China; [email protected] * Correspondence: [email protected] Abstract: Introduction: Accurate assessment of the corneal shape is important in cataract and refractive surgery, both in screening of candidates as well as for analyzing postoperative outcomes. Although corneal topography and tomography are widely used, it is common that these technologies are confused. The aim of this study was to present the current developments of these technologies and particularly distinguish between corneal topography and tomography. Methods: The PubMed, Web of Science and Embase databases were the main resources used to investigate the medical literature. The following keywords were used in various combinations: cornea, corneal, topography, tomography, Scheimpflug, Pentacam, optical coherence tomography. Results: Topography is the study of the shape of the corneal surface, while tomography allows a three-dimensional section of the cornea to be presented. Corneal topographers can be divided into large- and small-cone Placido-based devices, as well as devices with color-LEDs. For corneal tomography, scanning slit or Scheimpflug imaging and optical coherence tomography may be employed. In several devices, corneal topography and tomography have been successfully combined with tear-film analysis, aberrometry, optical biometry and anterior/posterior segment optical coherence tomography. -

Safety and Effectiveness of the UV-X System for Corneal Collagen Cross
Evaluation of two Riboflavin Dosing Regimens for Corneal Collagen Cross-Linking in Eyes with Progressive Keratoconus or Ectasia Protocol 2010-0243, Version: 15 Protocol Date: September 16, 2016 PHYSICIAN SPONSOR: Francis W. Price, Jr. MD CONFIDENTIAL Background This clinical protocol is designed to evaluate two riboflavin-dosing regimens for treatment of patients with progressive keratoconus or corneal ectasia using investigational technology that increases the cross linking of the corneal stroma using the photochemical interaction of UVA light with the chromophore riboflavin. In this treatment, the corneal stroma is saturated with riboflavin by irrigating the surface after removal of the corneal epithelium. The riboflavin-saturated cornea is then exposed to a uniform field of UVA light with a narrow bandwidth centered at 365 nm. The light is generated by an IROC UV-X irradiation system that creates a uniform 11 mm circle of UVA light. The device has a timer that allows a precise 30-minute exposure of the corneal tissues. The irradiation field of the UVX system produces UVA light with a uniform irradiance of 3 mj/cm2 at the corneal surface. The FDA has classified this technology as a combination product with two components. First is the UV-X light source, which has an LED source and is calibrated and rendered uniform by the use of an optical homogenizer. The second component is a riboflavin ophthalmic solution. This solution is used to saturate the corneal stroma prior to its photochemical activation. This combination product has been studied in a FDA-approved, randomized, placebo- controlled, multi-center trial that has enrolled patients. -

Refractive Surgery
I explore and get information from inside the eye, that´s my job. You can then go deeper into the diagnosis. You decide, I explore! FOR ACCURACY IN REFRACTIVE SURGERY ACE® is a technology that utilizes the power of high-resolution swept-source OCT imaging to provide the key corneal measurements. Optimizing the quality of the preoperative data provides more information to help you to improve the safety of your refractive surgery procedures.1 ACE® and the TECHNOLAS® TeneoTM 317 Model 2 offer solutions that will refine your results. Transform your daily surgical routine into an exciting day with a platform that brings together corneal topography and tomography and allowing data transfer between both devices. All corneal measurements are based on high-resolution swept-source OCT images KEY FUNCTIONS Corneal topography Corneal wavefront analysis Corneal tomography Differential maps Pachymetry Progression analysis Total corneal power Data transfer with TECHNOLAS® TENEOTM 317 Model 2 * All corneal measurements based on high- resolution swept-source OCT images 1. Muriël Doors et al. Value of optical coherence tomography for anterior segment surgery. J Cataract Refract Surg 2010; 36:1213–1229 Q 2010 ADVANCED CORNEAL EXPLORER HIGHLY CUSTOMIZABLE MAP LAYOUT Display up to 6 maps simultaneously, compare OD and OS, or perform an analysis over time. 12 different map types: Assess each patient’s corneal topography and Anterior and posterior axial or Pachymetry tomography, including tangential curvature Total corneal power curvature and elevation Anterior and posterior elevation maps of the anterior and Anterior and total corneal wavefront (best fit sphere and best fit torus) posterior surfaces. -
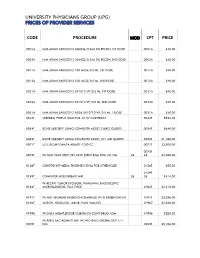
Code Procedure Cpt Price University Physicians Group
UNIVERSITY PHYSICIANS GROUP (UPG) PRICES OF PROVIDER SERVICES CODE PROCEDURE MOD CPT PRICE 0001A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 1ST DOSE 0001A $40.00 0002A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 2ND DOSE 0002A $40.00 0011A IMM ADMN SARSCOV2 100 MCG/0.5 ML 1ST DOSE 0011A $40.00 0012A IMM ADMN SARSCOV2 100 MCG/0.5 ML 2ND DOSE 0012A $40.00 0021A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 1ST DOSE 0021A $40.00 0022A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 2ND DOSE 0022A $40.00 0031A IMM ADMN SARSCOV2 AD26 5X10^10 VP/0.5 ML 1 DOSE 0031A $40.00 0042T CEREBRAL PERFUS ANALYSIS, CT W/CONTRAST 0042T $954.00 0054T BONE SURGERY USING COMPUTER ASSIST, FLURO GUIDED 0054T $640.00 0055T BONE SURGERY USING COMPUTER ASSIST, CT/ MRI GUIDED 0055T $1,188.00 0071T U/S LEIOMYOMATA ABLATE <200 CC 0071T $2,500.00 0075T 0075T PR TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL 26 26 $2,208.00 0126T CAROTID INT-MEDIA THICKNESS EVAL FOR ATHERSCLER 0126T $55.00 0159T 0159T COMPUTER AIDED BREAST MRI 26 26 $314.00 PR RECTAL TUMOR EXCISION, TRANSANAL ENDOSCOPIC 0184T MICROSURGICAL, FULL THICK 0184T $2,315.00 0191T PR ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT 0191T $2,396.00 01967 ANESTH, NEURAXIAL LABOR, PLAN VAG DEL 01967 $2,500.00 01996 PR DAILY MGMT,EPIDUR/SUBARACH CONT DRUG ADM 01996 $285.00 PR PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> 0200T NDL 0200T $5,106.00 PR PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> 0201T NDLS 0201T $9,446.00 PR INJECT PLATELET RICH PLASMA W/IMG 0232T HARVEST/PREPARATOIN 0232T $1,509.00 0234T PR TRANSLUMINAL PERIPHERAL ATHERECTOMY, RENAL