Risk-Taking by Incubating Common Goldeneyes and Hooded Mergansers ’
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Waterfowl in Iowa, Overview
STATE OF IOWA 1977 WATERFOWL IN IOWA By JACK W MUSGROVE Director DIVISION OF MUSEUM AND ARCHIVES STATE HISTORICAL DEPARTMENT and MARY R MUSGROVE Illustrated by MAYNARD F REECE Printed for STATE CONSERVATION COMMISSION DES MOINES, IOWA Copyright 1943 Copyright 1947 Copyright 1953 Copyright 1961 Copyright 1977 Published by the STATE OF IOWA Des Moines Fifth Edition FOREWORD Since the origin of man the migratory flight of waterfowl has fired his imagination. Undoubtedly the hungry caveman, as he watched wave after wave of ducks and geese pass overhead, felt a thrill, and his dull brain questioned, “Whither and why?” The same age - old attraction each spring and fall turns thousands of faces skyward when flocks of Canada geese fly over. In historic times Iowa was the nesting ground of countless flocks of ducks, geese, and swans. Much of the marshland that was their home has been tiled and has disappeared under the corn planter. However, this state is still the summer home of many species, and restoration of various areas is annually increasing the number. Iowa is more important as a cafeteria for the ducks on their semiannual flights than as a nesting ground, and multitudes of them stop in this state to feed and grow fat on waste grain. The interest in waterfowl may be observed each spring during the blue and snow goose flight along the Missouri River, where thousands of spectators gather to watch the flight. There are many bird study clubs in the state with large memberships, as well as hundreds of unaffiliated ornithologists who spend much of their leisure time observing birds. -

2020 MIGRATORY GAME BIRD SEASON PREVIEW Summary of Issues for Consideration
Comments may be sent to [email protected] 2020 MIGRATORY GAME BIRD SEASON PREVIEW Summary of Issues for Consideration: The majority of Vermont’s waterfowl season is driven by the federal framework for the Atlantic Flyway. Below are a few issues that must be decided for the 2020 hunting season. The Department would like the Board to consider the following: • Hold the liberal season allowed under the federal framework related to season lengths and daily bag limits. The Board has the option to be more conservative. • For the 2020 Duck Season. o Open the 2020 duck season on a Saturday, October 10. The change to opening every other year on a Saturday and Wednesday is consistent with hunter preferences of moving to alternating year approach for openings. o Any splits within seasons to create segments should be considered for the Lake Champlain and Interior Vermont zones. o Interior Zone: October 10 and run through December 8. o Lake Champlain Zone: October 10 - Nov. 1 and Nov. 21 - Dec. 27. • For the 2020 Goose Seasons o Open the resident Canada goose season September 1st and continue through September 25. o Open the migratory Canada goose season on October 10. o Opening the Snow goose season on October 1. • Hold youth hunting weekend – September 26-27. • Hold woodcock/snipe season: October 1- November 14. • Board has option to have a two-day active duty/veterans hunt. Currently, the Department does not propose doing this. Background In 2016 the Department began fully reviewing the migratory game bird season options with the Board without being under a very short time constraint. -

Texas Wildlife Identification Guide: a Guide to Game Animals, Game
texas parks and wildlife TEXAS WILDLIFE IDENTIFICATION GUIDE A guide to game animals, game birds, furbearers and other wildlife of Texas. INTRODUCTION TEXAS game animals, game birds, furbearers and other wildlife are important for many reasons. They provide countless hours of viewing and recreational opportunities.They benefit the Texas economy through hunting and “nature tourism” such as birdwatching. Commercial businesses that provide birdseed, dry corn and native landscaping may be devoted solely to attracting many of the animals found in this book. Local hunting and trapping economies, guiding operations and hunting leases have prospered because of the abun- dance of these animals in Texas.The Texas Parks and Wildlife Department benefits because of hunting license sales, but it uses these funds to research, manage and pro- tect all wildlife populations – not just game animals. Game animals provide humans with cultural, social, aesthetic and spiritual pleasures found in wildlife art, taxi- dermy and historical artifacts. Conservation organizations dedicated to individual species such as quail, turkey and deer, have funded thousands of wildlife projects throughout North America, demonstrating the mystique game animals have on people. Animals referenced in this pocket guide exist because their habitat exists in Texas. Habitat is food, cover, water and space, all suitably arranged.They are part of a vast food chain or web that includes thousands more species of wildlife such as the insects, non-game animals, fish and i rare/endangered species. Active management of wild landscapes is the primary means to continue having abundant populations of wildlife in Texas. Preservation of rare and endangered habitat is one way of saving some species of wildlife such as the migratory whooping crane that makes Texas its home in the winter. -

Nest Box Guide for Waterfowl Nest Box Guide for Waterfowl Copyright © 2008 Ducks Unlimited Canada ISBN 978-0-9692943-8-2
Nest Box Guide for Waterfowl Nest Box Guide For Waterfowl Copyright © 2008 Ducks Unlimited Canada ISBN 978-0-9692943-8-2 Any reproduction of this present document in any form is illegal without the written authorization of Ducks Unlimited Canada. For additional copies please contact the Edmonton DUC office at (780)489-2002. Published by: Ducks Unlimited Canada www.ducks.ca Acknowledgements Photography provided by : Ducks Unlimited Canada (DUC), Jim Potter (Alberta Conservation Association (ACA)), Darwin Chambers (DUC), Jonathan Thompson (DUC), Lesley Peterson (DUC contractor), Sherry Feser (ACA), Gordon Court ( p 16 photo of Pygmy Owl), Myrna Pearman ,(Ellis Bird Farm), Bryan Shantz and Glen Rowan. Portions of this booklet are based on a Nest Box Factsheet prepared by Jim Potter (ACA) and Lesley Peterson (DUC contractor). Myrna Pearman provided editorial comment. Table of Contents Table of Contents Why Nest Boxes? ......................................................................................................1 Natural Cavities ......................................................................................................................................2 Identifying Wildlife Species That Use Your Nest Boxes .....................................3 Waterfowl ..................................................................................................................4 Common Goldeneye .........................................................................................................................5 Barrow’s Goldeneye -

Derivation of Non-Breeding Duck
North American Waterfowl Management Plan Science Support Team Technical Report No. 2019–01 Derivation of Regional, Non-breeding Duck Population Abundance Objectives to Inform Conservation Planning in North America — 2019 Revision Kathy K. Fleming U.S. Fish and Wildlife Service, Division of Migratory Bird Management Michael K. Mitchell Ducks Unlimited, Inc. Southern Regional Office Michael G. Brasher Ducks Unlimited, Inc., Gulf Coast Joint Venture John M. Coluccy Ducks Unlimited, Inc., Great Lakes and Atlantic Regional Office J. Dale James Ducks Unlimited, Inc. Southern Regional Office Mark J. Petrie Ducks Unlimited, Inc., Western Regional Office, Pacific Coast Joint Venture Dale D. Humburg Ducks Unlimited, Inc., National Headquarters Gregory J. Soulliere U. S. Fish and Wildlife Service, Upper Mississippi / Great Lakes Joint Venture ABSTRACT During the early 2000s, a methodology was developed to derive regional non-breeding population abundance objectives from continental abundance estimates (M. Koneff, USFWS, unpublished data). This information was foundational to North American Waterfowl Management Plan (NAWMP) Joint Venture (JV) habitat conservation planning and implementation for non-breeding waterfowl, especially wintering ducks. The 2012 NAWMP Revision and its amended population objectives motivated JVs to begin updating their waterfowl implementation plans. Fleming et al. (2017) revisited the initial work to derive non- breeding abundance objectives and developed an updated approach. Although Fleming et al. (2017) made use of the least biased and most geographically consistent datasets, they identified outstanding issues to be resolved before the derivation technique could be effectively applied across all regions of North America. We updated the work of Fleming et al. (2017) by addressing 3 of those issues. -

And Bufflehead (Bucephala Albeola) in Alberta, Canada
Copyright © 2011 by the author(s). Published here under license by the Resilience Alliance. Corrigan, R. M., G. J. Scrimgeour, and C. Paszkowski. 2011. Nest boxes facilitate local-scale conservation of common goldeneye (Bucephala clangula) and bufflehead (Bucephala albeola) in Alberta, Canada. Avian Conservation and Ecology 6(1): 1. http://dx.doi.org/10.5751/ACE-00435-060101 Research Papers Nest Boxes Facilitate Local-Scale Conservation of Common Goldeneye (Bucephala clangula) and Bufflehead (Bucephala albeola) in Alberta, Canada Les nichoirs favorisent la conservation à l’échelle locale du Garrot à œil d’or (Bucephala clangula) et du Petit Garrot (Bucephala albeola) en Alberta, Canada Robert M. Corrigan 1,2, Garry J. Scrimgeour 3, and Cynthia Paszkowski 1 ABSTRACT. We tested the general predictions of increased use of nest boxes and positive trends in local populations of Common Goldeneye (Bucephala clangula) and Bufflehead (Bucephala albeola) following the large-scale provision of nest boxes in a study area of central Alberta over a 16-year period. Nest boxes were rapidly occupied, primarily by Common Goldeneye and Bufflehead, but also by European Starling (Sturnus vulgaris). After 5 years of deployment, occupancy of large boxes by Common Goldeneye was 82% to 90% and occupancy of small boxes by Bufflehead was 37% to 58%. Based on a single-stage cluster design, experimental closure of nest boxes resulted in significant reductions in numbers of broods and brood sizes produced by Common Goldeneye and Bufflehead. Occurrence and densities of Common Goldeneye and Bufflehead increased significantly across years following nest box deployment at the local scale, but not at the larger regional scale. -
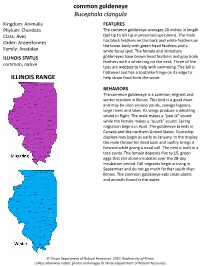
Common Goldeneye Bucephala Clangula ILLINOIS RANGE
common goldeneye Bucephala clangula Kingdom: Animalia FEATURES Phylum: Chordata The common goldeneye averages 20 inches in length Class: Aves (tail tip to bill tip in preserved specimen). The male Order: Anseriformes has black feathers on the back and white feathers on the lower body with green head feathers and a Family: Anatidae white facial spot. The female and immature ILLINOIS STATUS goldeneyes have brown head feathers and gray body feathers with a white ring on the neck. Three of the common, native toes are webbed to help with swimming. The bill is flattened and has a toothlike fringe on its edge to ILLINOIS RANGE help strain food from the water. BEHAVIORS The common goldeneye is a common migrant and winter resident in Illinois. This bird is a good diver and may be seen around ponds, sewage lagoons, large rivers and lakes. Its wings produce a whistling sound in flight. The male makes a “pee-ik” sound while the female makes a “quack” sound. Spring migration begins in April. The goldeneye breeds in Canada and the northern United States. Courtship displays may begin as early as January. In the display the male throws his head back and swiftly brings it forward while giving a nasal call. The nest is built in a tree cavity. The female deposits five to 15, green eggs that she alone incubates over the 28-day incubation period. Fall migrants begin arriving in September and do not go much further south than Illinois. The common goldeneye eats small plants and animals found in the water. © Illinois Department of Natural Resources. -

Summary of National Hunting Regulations: United Kingdom
Summary of National Hunting Regulations: United Kingdom Updated in October 2016 HUNTING AND TRAPPING LEGISLATION/ RESOURCES Name of main legislation: In the UK hunting law is a national issue, therefore several hunting laws exist: Wildlife and Countryside Act 1981 for England, Scotland and Wales; Wildlife (Northern Ireland) Order 1985 Year of publication: see above Supporting legislation: Legislation updates: In England and Wales the law has been amended by the Countryside and Rights of Way Act 2000 and in Scotland by the Nature Conservation (Scotland) Act 2004. Hunting legislation (web link): http://www.legislation.gov.uk/browse Authority in charge of controlling hunting (web link): Home Office (Police) UK National Wildlife Crime Unit http://www.nwcu.police.uk/ Major inconsistencies or loopholes detected (if yes please describe): The UK lacks a system of licensing for hunting, with no statutory limits applied to hunting bags, or statutory requirement to submit returns. Questions have been raised about consequences for the UK’s compliance with principles under Article 7 of the Birds Directive, including “wise use”, “ecologically balanced control” and requirement not to “jeopardise conservation efforts” for huntable or non- huntable species. The lack of a licensing system also limits the capacity of authorities to apply restrictions on hunting in response to incidents of wildlife crime. Note: the devolved Scottish Government have commissioned a review of game licensing systems in Europe, which is expected to report this year. Derogations (on EU Birds Directive and/or Bern Convention): None HUNTING AND TRAPPING: METHODS AND RESTRICTIONS Legal methods/restrictions Notes - Certain game bird species can be hunt during the open shooting season (see below) - There is no requirement to hold a hunting licence. -

Game Birds of the World Species List
Game Birds of North America GROUP COMMON NAME SCIENTIFIC NAME QUAIL Northern bobwhite quail Colinus virginianus Scaled quail Callipepla squamata Gambel’s quail Callipepla gambelii Montezuma (Mearns’) quail Cyrtonyx montezumae Valley quail (California quail) Callipepla californicus Mountain quail Oreortyx pictus Black-throated bobwhite quail Colinus nigrogularis GROUSE Ruffed grouse Bonasa umbellus Spruce grouse Falcipennis canadensis Blue grouse Dendragapus obscurus (Greater) sage grouse Centrocercus urophasianus Sharp-tailed grouse Tympanuchus phasianellus Sooty grouse Dendragapus fuliginosus Gunnison sage-grouse Centrocercus minimus PARTRIDGE Chukar Alectoris chukar Hungarian partridge Perdix perdix PTARMIGAN Willow ptarmigan Lagopus lagopus Rock ptarmigan Lagopus mutus White-tailed ptarmigan Logopus leucurus WOODCOCK American woodcock Scolopax minor SNIPE Common snipe Gallinago gallinago PHEASANT Pheasant Phasianus colchicus DOVE Mourning dove Zenaida macroura White-winged dove Zenaida asiatica Inca dove Columbina inca Common ground gove Columbina passerina White-tipped dove GROUP COMMON NAME SCIENTIFIC NAME DIVING DUCK Ruddy duck Oxyuia jamaiceusis Tufted duck Aythya fuliqule Canvas back Aythya valisineoia Greater scaup Aythya marila Surf scoter Melanitta persicillata Harlequin duck Histrionicus histrionicus White-winged scoter Melanitta fusca King eider Somateria spectabillis Common eider Somateria mollissima Barrow’s goldeneye Buchephala islandica Black scoter Melanitta nigra amerieana Redhead Aythya americana Ring-necked duck aythya -

Common Goldeneye (Bucephala Clangula) French: Garrot À Oeil D'or
Sea Duck Information Series Common Goldeneye (Bucephala clangula) French: Garrot à oeil d'or Description Common goldeneyes are chunky, medium sized sea ducks. Males are 45-50 cm (17 in.) long and weigh about 1000 g (2.2 lbs.) and females are 40-50 cm (15 in.) and 800 g (1.8 lbs.). Both sexes have a bright yellow iris, hence the name “goldeneye”. Males in breeding plumage (October to June) have an iridescent greenish-black head and a bright oval white patch behind the bill. Their white belly, breast, flanks, and neck contasts greatly with the otherwise black feathering of their back and tail. It can be distinguished readily from Barrow’s goldeneye by the oval patch behind bill versus the crescent shape of Barrow’s. The bill is slightly longer and more wedge-shaped and the forehead rises more gradually than Barrow’s. Females have a chocolate-brown Common Goldeneye pair head, dark gray back and tail, and white belly, breast, and flanks. Their Habitat and Habits nearby. The female often abandons bill is black and tipped with yellow. Common goldeneyes are often the brood before they can fly at about Female common goldeneyes are the last waterfowl to move south in 60 days. difficult to tell apart from Barrow’s the fall and one of the first species Mortality of ducklings is highest females. Immature males are to migrate north in spring, arriving during the first two weeks of life; difficult to distinguish from females. as soon as the first open water is causes of death include adverse In flight, the inner wings of both available. -

Choice of Nest Boxes by Common Goldeneyes in Ontario
Wilson Bull., 92(4), 1980, pp. 497-505 CHOICE OF NEST BOXES BY COMMON GOLDENEYES IN ONTARIO HARRY G. LUMSDEN, R. E. PAGE AND M. GAUTHIER Nest boxes were hung on trees for Common Goldeneyes (Bucephala clangula) in Scandinavia over 240 years ago (Linnaeus in Phillips 1925, Lloyd 1854) to provide a ready source of eggs for human consumption. The use of boxes to increase stocks is more recent and has been practiced in Europe as well as North America. Few data exist in North America on the size of natural cavities chosen by goldeneyes. Sixteen cavities found in New Brunswick (Prince 1968) had an average inside diameter of 20.6 ? 4.1 cm, a depth of 46.2 + 19.6 cm, and the average size of the entrance hole was 22.4 -+ 16.3 cm long X 11.4 + 3.6 cm wide. Ten of these 16 cavities were open at the top like a chimney. Siren (1951) tested hollow pine logs in Finland with a variety of dimensions and recommended specific measurements. Palmer (1976) also made recommendations. These are summarized in Table 1 with the mea- surements of nest boxes used by 5 other investigators in their studies of nesting goldeneyes. The purpose of this paper is to describe the results of selection experiments in which goldeneyes were presented with boxes with a variety of features. MATERIALS AND METHODS The work was started in 1974 and continues on 3 study areas in Ontario: Elk Lake (47”44N,’ 80”2OW);’ the Englehart River including Robillard, Kinogami and Kushog lakes, known col- lectively as Long Lake, near the village of Charlton (47”48N,’ 79”59W);’ and on the Mattagami and Muskego rivers west and south of Smooth Rock Falls (49”17N,’ 81”38W).’ We used nest boxes made from 1.27 cm sheeting grade plywood, with a relatively rough surface. -

Sustainable Management of Migratory European Ducks: Finding Model Species Author(S): Sari Holopainen, Céline Arzel, Johan Elmberg, Anthony D
Sustainable management of migratory European ducks: finding model species Author(s): Sari Holopainen, Céline Arzel, Johan Elmberg, Anthony D. Fox, Matthieu Guillemain, Gunnar Gunnarsson, Petri Nummi, Kjell Sjöberg, Veli-Matti Väänänen, Mikko Alhainen and Hannu Pöysä Source: Wildlife Biology, 2018() Published By: Nordic Board for Wildlife Research https://doi.org/10.2981/wlb.00336 URL: http://www.bioone.org/doi/full/10.2981/wlb.00336 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Wildlife Biology 2018: wlb.00336 doi: 10.2981/wlb.00336 © 2018 The Authors. This is an Open Access article Subject Editor: Olafur Nielsen. Editor-in-Chief: Ilse Storch. Accepted 13 February 2018 Sustainable management of migratory European ducks: finding model species Sari Holopainen, Céline Arzel, Johan Elmberg, Anthony D. Fox, Matthieu Guillemain, Gunnar Gunnarsson, Petri Nummi, Kjell Sjöberg, Veli-Matti Väänänen, Mikko Alhainen and Hannu Pöysä S.