Report of Coral Diseases in the Reef Flats of Chetlat Island, Lakshadweep
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lakshadweep Action Plan on Climate Change 2012 2012 333333333333333333333333
Lakshadweep Action Plan on Climate Change 2012 2012 333333333333333333333333 LAKSHADWEEP ACTION PLAN ON CLIMATE CHANGE (LAPCC) UNION TERRITORY OF LAKSHADWEEP i SUPPORTED BY UNDP Lakshadweep Action Plan on Climate Change 2012 LAKSHADWEEP ACTION PLAN ON CLIMATE CHANGE (LAPCC) Department of Environment and Forestry Union Territory of Lakshadweep Supported by UNDP ii Lakshadweep Action Plan on Climate Change 2012 Foreword 2012 Climate Change (LAPCC) iii Lakshadweep Action Plan on Lakshadweep Action Plan on Climate Change 2012 Acknowledgements 2012 Climate Change (LAPCC) iv Lakshadweep Action Plan on Lakshadweep Action Plan on Climate Change 2012 CONTENTS FOREWORD .......................................................................................................................................... III ACKNOWLEDGEMENTS .................................................................................................................... IV EXECUTIVE SUMMARY .................................................................................................................. XIII PART A: CLIMATE PROFILE .............................................................................................................. 1 1 LAKSHADWEEP - AN OVERVIEW ............................................................................................. 2 1.1 Development Issues and Priorities .............................................................................................................................. 3 1.2 Baseline Scenario of Lakshadweep ............................................................................................................................ -

Microbiomes of Gall-Inducing Copepod Crustaceans from the Corals Stylophora Pistillata (Scleractinia) and Gorgonia Ventalina
www.nature.com/scientificreports OPEN Microbiomes of gall-inducing copepod crustaceans from the corals Stylophora pistillata Received: 26 February 2018 Accepted: 18 July 2018 (Scleractinia) and Gorgonia Published: xx xx xxxx ventalina (Alcyonacea) Pavel V. Shelyakin1,2, Sofya K. Garushyants1,3, Mikhail A. Nikitin4, Sofya V. Mudrova5, Michael Berumen 5, Arjen G. C. L. Speksnijder6, Bert W. Hoeksema6, Diego Fontaneto7, Mikhail S. Gelfand1,3,4,8 & Viatcheslav N. Ivanenko 6,9 Corals harbor complex and diverse microbial communities that strongly impact host ftness and resistance to diseases, but these microbes themselves can be infuenced by stresses, like those caused by the presence of macroscopic symbionts. In addition to directly infuencing the host, symbionts may transmit pathogenic microbial communities. We analyzed two coral gall-forming copepod systems by using 16S rRNA gene metagenomic sequencing: (1) the sea fan Gorgonia ventalina with copepods of the genus Sphaerippe from the Caribbean and (2) the scleractinian coral Stylophora pistillata with copepods of the genus Spaniomolgus from the Saudi Arabian part of the Red Sea. We show that bacterial communities in these two systems were substantially diferent with Actinobacteria, Alphaproteobacteria, and Betaproteobacteria more prevalent in samples from Gorgonia ventalina, and Gammaproteobacteria in Stylophora pistillata. In Stylophora pistillata, normal coral microbiomes were enriched with the common coral symbiont Endozoicomonas and some unclassifed bacteria, while copepod and gall-tissue microbiomes were highly enriched with the family ME2 (Oceanospirillales) or Rhodobacteraceae. In Gorgonia ventalina, no bacterial group had signifcantly diferent prevalence in the normal coral tissues, copepods, and injured tissues. The total microbiome composition of polyps injured by copepods was diferent. -

Agatti Island, UT of Lakshadweep
Socioeconomic Monitoring for Coastal Managers of South Asia: Field Trials and Baseline Surveys Agatti Island, UT of Lakshadweep Project completion Report: NA10NOS4630055 Project Supervisor : Vineeta Hoon Site Coordinators: Idrees Babu and Noushad Mohammed Agatti team: Amina.K, Abida.FM, Bushra M.I, Busthanudheen P.K, Hajarabeebi MC, Hassan K, Kadeeshoma C.P, Koyamon K.G, Namsir Babu.MS, Noorul Ameen T.K, Mohammed Abdul Raheem D A, Shahnas beegam.k, Shahnas.K.P, Sikandar Hussain, Zakeer Husain, C.K, March 2012 This volume contains the results of the Socioeconomic Assessment and monitoring project supported by IUCN/ NOAA Prepared by: 1. The Centre for Action Research on Environment Science and Society, Chennai 600 094 2. Lakshadweep Marine Research and Conservation Centre, Kavaratti island, U.T of Lakshadweep. Citation: Vineeta Hoon and Idrees Babu, 2012, Socioeconomic Monitoring and Assessment for Coral Reef Management at Agatti Island, UT of Lakshadweep, CARESS/ LMRCC, India Cover Photo: A reef fisherman selling his catch Photo credit: Idrees Babu 2 Table of Contents Executive Summary 7 Acknowledgements 8 Glossary of Native Terms 9 List of Acronyms 10 1. Introduction 11 1.1 Settlement History 11 1.2 Dependence on Marine Resources 13 1.3 Project Goals 15 1.4 Report Chapters 15 2. Methodology of Project Execution 17 2.1 SocMon Workshop 17 2.2 Data Collection 18 2.3 Data Validation 20 3. Site Description and Island Infrastructure 21 3.1 Site description 23 3.2. Community Infrastructure 25 4. Community Level Demographics 29 4.1 Socio cultural status 29 4.2 Land Ownership 29 4.3 Demographic characteristics 30 4.4 Household size 30 4.5. -

Scleractinia Fauna of Taiwan I
Scleractinia Fauna of Taiwan I. The Complex Group 台灣石珊瑚誌 I. 複雜類群 Chang-feng Dai and Sharon Horng Institute of Oceanography, National Taiwan University Published by National Taiwan University, No.1, Sec. 4, Roosevelt Rd., Taipei, Taiwan Table of Contents Scleractinia Fauna of Taiwan ................................................................................................1 General Introduction ........................................................................................................1 Historical Review .............................................................................................................1 Basics for Coral Taxonomy ..............................................................................................4 Taxonomic Framework and Phylogeny ........................................................................... 9 Family Acroporidae ............................................................................................................ 15 Montipora ...................................................................................................................... 17 Acropora ........................................................................................................................ 47 Anacropora .................................................................................................................... 95 Isopora ...........................................................................................................................96 Astreopora ......................................................................................................................99 -

Odam – the Quintessential Sewn Boat of India Odam – L’Essence Du Bateau Cousu De L’Inde
Archaeonautica L’archéologie maritime et navale de la préhistoire à l’époque contemporaine 20 | 2018 De re navali : Pérégrinations nautiques entre Méditerranée et océan Indien Odam – the quintessential sewn boat of India Odam – L’essence du bateau cousu de l’Inde Lotika Varadarajan Electronic version URL: http://journals.openedition.org/archaeonautica/594 DOI: 10.4000/archaeonautica.594 ISSN: 2117-6973 Publisher CNRS Éditions Printed version Date of publication: 6 December 2018 Number of pages: 209-221 ISBN: 978-2-271-12263-6 ISSN: 0154-1854 Electronic reference Lotika Varadarajan, « Odam – the quintessential sewn boat of India », Archaeonautica [Online], 20 | 2018, Online since 30 April 2020, connection on 30 April 2020. URL : http://journals.openedition.org/ archaeonautica/594 ; DOI : https://doi.org/10.4000/archaeonautica.594 Archaeonautica ODAM – THE QUINTESSENTIAL SEWN BOAT OF INDIA Lotika VARADARAJAN Abstract ODAM – l’ESSENCE DU BATEAU COUSU DE L’INDE The article opens with a preliminary introduction to the trade Résumé routes that existed in antiquity and the role of Indian trade as L’article s’ouvre sur une introduction relative aux routes commer- regards these routes. India could have played a passive role and ciales de l’Antiquité et sur le rôle tenu par le commerce indien au sein allowed foreign merchants to handle her commerce. This did de ces routes. L’Inde aurait pu jouer un rôle passif et ainsi permettre not happen as the sub-continent had the wherewithal to play aux commerçants étrangers de gérer son commerce. Cela ne s’est an effective role. This article will concentrate on the ships that pas produit car le sous-continent avait les moyens de jouer un rôle de handled this trade. -

Community Assembly and Functions of the Coral Skeleton Microbiome
Beneath the surface: community assembly and functions of the coral skeleton microbiome Francesco Ricci1, Vanessa Rossetto Marcelino2, Linda L. Blackall1, Michael Kühl3,4, Monica Medina5, Heroen Verbruggen1# 1 - School of BioSciences, University of Melbourne, Parkville 3010, Australia 2 - Marie Bashir Institute for Infectious Diseases and Biosecurity, Sydney Medical School, Westmead Clinical School, The University of Sydney, Sydney, NSW 2006, Australia 3 - Marine Biological Section, University of Copenhagen, Strandpromenaden 5, DK-3000 Helsingør, Denmark 4 - Climate Change Cluster, University of Technology Sydney, Ultimo, NSW 2007, Australia 5 - Pennsylvania State University, University Park, PA 16802, USA # - corresponding author: [email protected] Author email addresses: Francesco Ricci ([email protected]), Vanessa Rossetto Marcelino ([email protected]), Linda Blackall ([email protected]), Michael Kühl ([email protected]), Monica Medina ([email protected]), Heroen Verbruggen ([email protected]) Abstract Coral microbial ecology is a burgeoning field, driven by the urgency of understanding coral health and slowing reef loss due to climate change. Coral resilience depends on its microbiota, and both the tissue and the underlying skeleton are home to a rich biodiversity of eukaryotic, bacterial and archaeal species that form an integral part of the coral holobiont. New techniques now enable detailed studies of the endolithic habitat, and our knowledge of the skeletal microbial community and its eco-physiology is increasing rapidly, with multiple lines of evidence for the importance of the skeletal microbiota in coral health and functioning. Here, we review the roles these organisms play in the holobiont, including nutritional exchanges with the coral host and decalcification of the host skeleton. -

1 Government of India Lakshadweep Administration (DEPARTMENT OF
THE LAKSHADWEEP GAZETTE EXTRAORDINARY 1 VOL. LVI. No. 28 THURSDAY 15th OCTOBER, 2020 / 23rd ASVINA, 1942 (SAKA) Government of India Lakshadweep Administration (DEPARTMENT OF ENVIRONMENT AND FOREST) Kavaratti Island, Dated : 14-10-2020. NOTIFICATION F.No. 2/22/2020-E&F:- The Administrator, Union Territory of Lakshadweep Administration is pleased to publish the Lakshadweep Forest Code-2020 of the Department of Environment and Forest, Union Territory of Lakshadweep Administration and is declared as the Official Forest Code of the department with effect from the date of publication of this notification in the Official Gazette. This is issued with the approval of Hon’ble Administrator vide Diary No. 1752, dated 07-10-2020. Sd/- (DAMODHAR A.T., IFS) Secretary, Environment & Forest and Chief Wildlife Warden. LGP.Kvt. G-1389/10 -20/50 PRICE: 75 PAISE 2 THE LAKSHADWEEP GAZETTE EXTRAORDINARY CHAPTER – I ORGANISATION OF THE DEPARTMENT OF ENVIRONMENT AND FOREST 1 – GENERAL 1.1 Introduction Natural ecosystems are a dynamic ecosystem consisting of plants, animals & microorganisms safeguarding the ecological security of the nation. It provides various ecosystem services essential for the very survival of the human beings. The aim of the Department of Environment & Forest in UT of Lakshadweep is department and services ensure environmental stability and maintenance of ecological balance including atmospheric equilibrium which are vital for sustenance ofall life forms, human, animals and plants. This policy will be instrumental in strengthening ecological security, sustainable ecological management, and participatory management. This also ensures to safeguard the ecological and livelihood security of people, of the present and future generations, based on sustainable management of the ecosystem services thereby the stability of the fragile eco-systems. -

20 Year Perspective Plan for Tourism Development In
20 YEAR PERSPECTIVE PLAN FOR TOURISM DEVELOPMENT IN LAKSHADWEEP ISLANDS (FINAL REPORT) Prepared for DEPARTMENT OF TOURISM GOVERNMENT OF INDIA NEW DELHI JANUARY 2003 TATA ECONOMIC CONSULTANCY SERVICES VI-A, ELDORADO, 112, NUNGAMBAKKAM HIGH ROAD CHENNAI - 600 034 CONTENTS CHAPTER PAGE TITLE NO. NO. – EXECUTIVE SUMMARY 1-28 I INTRODUCTION 1-10 II DEMOGRAPHIC TRENDS AND ECONOMIC ACTIVITIES 1-8 III THE TRANSPORTATION SECTOR 1-19 IV OTHER PHYSICAL AND SOCIAL INFRASTRUCTURE 1-12 V PRESENT TOURISM SCENARIO IN THE ISLANDS 1-23 VI HUMAN RESOURCES DEVELOPMENT 1-5 VII TOURISM DEVELOPMENT STRATEGY AND 1-72 PROMOTIONAL PROGRAMME VIII ENVIRONMENTAL ISSUES 1-13 IX ECONOMIC BENEFITS 1-4 X MARKET DEVELOPMENT STRATEGY 1-7 XI FINANCIAL IMPLICATIONS 1-6 XII PROJECT BRIEFS 1-32 i.exe EXHIBITS EXHIBIT PAGE TITLE NO. NO. 2.1 LIST OF ISLANDS / ISLETS IN THE C.D. BLOCKS 7 2.2 POPULATION DISTRIBUTION AND OTHER RELATED 8 FACTORS 8.1 ENVIRONMENT MATRIX - POWER GENERATION 9 8.2 ENVIRONMENT MATRIX - PORT ACTIVITIES 10 8.3 ENVIRONMENT MATRIX - TOURISM 11 8.4 ENVIRONMENT MATRIX - PWD 12 8.5 ENVIRONMENT MATRIX - PRINTING 13 i.exe EXECUTIVE SUMMARY BACKGROUND 1. The central theme of the development process as envisaged in this Perspective Plan is to promote tourism potential as comprehensive as possible in the next span of 20 years. The basic premises under which the Tourism Perspective Plan is prepared and presented in this report involved: To identify and develop tourism assets in the islands in an optimum manner such that these can be enjoyed by both domestic and international markets and also in a manner that brings substantial benefits to the economy. -

COVID 19 Situation in Lakshadweep Islands
Vol. * XXXVI. No. 09 * KAVARATTI *WEDNESDAY * 23 JUNE 2021. Price Rs. 2. Hon'ble Administrator Collector and Chairman Shri Praful Patel District Disaster Manage- A COVID patient evacuating visited Lakshadweep and ment Authority Shri S. from Chetlat by Ambulance discussed various Asker Ali, IAS chairing a helicopter. developmental activities of meeting at Agatti Island. the territory. COVID 19 Situation in Hon'ble Administrator visited Lakshadweep Islands Lakshadweep 28th December, 2020, Collector said. Following the revised SOP, livelihood activities resumed in the islands. 945 tourists visited the islands in the five months since SOP was revised. Mechanized sailing vessels operated in the islands for 380 times. Consequently, construction activities A view from the COVID test at Kavaratti. started in the islands. Kavaratti : Due to the considering the genuine Flight operations were COVID 19 pandemic, requirement of the local also resumed. During this Lakshadweep islands population in terms of period, 35,170 people had highly restricted livelihood activities, came from mainland to Hon'ble Administrator Shri Praful Patel chairing a meeting conducted at movement of men and developmental islands majority of them Secretariat Conference Hall, Kavaratti. are local population and material from the mainland requirements of the local people in the to safeguard and protect considered, then 92,231 people travelled by to the islands and vice Administration and the islands also contributed its citizens from the Lakshadweep ships and HSC within versa from March prevailing MHA Guidelines significantly to the rise of pandemic. As a result of Administration has Lakshadweep islands, he to December 2020. during that time. As per COVID 19 cases in such continuous efforts, vaccinated 97.16% of its said. -

Downloaded from the NCBI Genome Database, Dehyde, and Post-Fixed in 1% Oso4
Yang et al. Microbiome (2019) 7:3 https://doi.org/10.1186/s40168-018-0616-z RESEARCH Open Access Metagenomic, phylogenetic, and functional characterization of predominant endolithic green sulfur bacteria in the coral Isopora palifera Shan-Hua Yang1,2,3,4, Kshitij Tandon1,5,6, Chih-Ying Lu1, Naohisa Wada1, Chao-Jen Shih7, Silver Sung-Yun Hsiao8,9, Wann-Neng Jane10, Tzan-Chain Lee1, Chi-Ming Yang1, Chi-Te Liu11, Vianney Denis12, Yu-Ting Wu13, Li-Ting Wang7, Lina Huang7, Der-Chuen Lee8, Yu-Wei Wu14, Hideyuki Yamashiro2 and Sen-Lin Tang1* Abstract Background: Endolithic microbes in coral skeletons are known to be a nutrient source for the coral host. In addition to aerobic endolithic algae and Cyanobacteria, which are usually described in the various corals and form a green layer beneath coral tissues, the anaerobic photoautotrophic green sulfur bacteria (GSB) Prosthecochloris is dominant in the skeleton of Isopora palifera. However, due to inherent challenges in studying anaerobic microbes in coral skeleton, the reason for its niche preference and function are largely unknown. Results: This study characterized a diverse and dynamic community of endolithic microbes shaped by the availability of light and oxygen. In addition, anaerobic bacteria isolated from the coral skeleton were cultured for the first time to experimentally clarify the role of these GSB. This characterization includes GSB’s abundance, genetic and genomic profiles, organelle structure, and specific metabolic functions and activity. Our results explain the advantages endolithic GSB receive from living in coral skeletons, the potential metabolic role of a clade of coral- associated Prosthecochloris (CAP) in the skeleton, and the nitrogen fixation ability of CAP. -
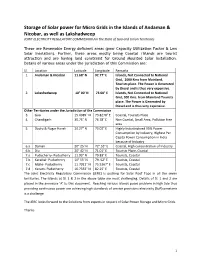
Storage of Solar Power for Micro Grids in the Islands of Andaman
Storage of Solar power for Micro Grids in the Islands of Andaman & Nicobar, as well as Lakshadweep JOINT ELECTRICITY REGULATORY COMMISSION For the State of Goa and Union Territories These are Renewable Energy deficient areas (poor Capacity Utilization Factor & Low Solar Insolation). Further, these areas mostly being Coastal /Islands are tourist attraction and are having land constraint for Ground Mounted Solar Installation. Details of various areas under the jurisdiction of this Commission are: Sl. Location Latitude Longitude Remarks 1. Andaman & Nicobar 11.68° N 92.77° E Islands, Not Connected to National Grid, 1000 Kms from Mainland, Tourists place. The Power is Generated by Diesel and is thus very expensive. 2. Lakashadweep 10° 00' N 73.00° E Islands, Not Connected to National Grid, 300 Kms. from Mainland Tourists place. The Power is Generated by Diesel and is thus very expensive. Other Territories under the Jurisdiction of the Commission 3. Goa 15.4989° N 73.8278° E Coastal, Tourists Place 4. Chandigarh 30.75° N 76.78° E Non Coastal, Small Area, Pollution free area 5. Dadra & Nagar Haveli 20.27° N 73.02° E Highly Industrialized 95% Power Consumption by Industry, Highest Per Capita Power Consumption in India because of Industry 6.a Daman 20° 25' N 72°.53° E Coastal, High concentration of Industry 6.b Diu 20° 42' N 71.01° E Tourists Place, Coastal 7.a Puducherry- Puducherry 11.93° N 79.83° E Tourists, Coastal 7.b Karaikal- Puducherry 10° 55' N 79. 52° E Tourists, Coastal 7.c Mahe- Puducherry 11.7011° N 75.5367° E Tourists, Coastal 7.d Yanam- Puducherry 16.7333° N 82.25° E Tourists, Coastal The Joint Electricity Regulatory Commission (JERC) is pushing for Solar Roof Tops in all the seven territories. -

Investigating the Driving Mechanisms Behind Differences in Bleaching
Florida International University FIU Digital Commons FIU Electronic Theses and Dissertations University Graduate School 6-15-2015 Investigating the Driving Mechanisms Behind Differences in Bleaching and Disease Susceptibility Between Two Scleractinian Corals, Pseudodiploria Strigosa and Diploria Labyrinthiformis Zoe A. Pratte Florida International University, [email protected] DOI: 10.25148/etd.FIDC000082 Follow this and additional works at: https://digitalcommons.fiu.edu/etd Part of the Aquaculture and Fisheries Commons, Bacteriology Commons, Environmental Microbiology and Microbial Ecology Commons, Genomics Commons, Immunity Commons, Marine Biology Commons, and the Other Animal Sciences Commons Recommended Citation Pratte, Zoe A., "Investigating the Driving Mechanisms Behind Differences in Bleaching and Disease Susceptibility Between Two Scleractinian Corals, Pseudodiploria Strigosa and Diploria Labyrinthiformis" (2015). FIU Electronic Theses and Dissertations. 2217. https://digitalcommons.fiu.edu/etd/2217 This work is brought to you for free and open access by the University Graduate School at FIU Digital Commons. It has been accepted for inclusion in FIU Electronic Theses and Dissertations by an authorized administrator of FIU Digital Commons. For more information, please contact [email protected]. FLORIDA INTERNATIONAL UNIVERSITY Miami, Florida INVESTIGATING THE DRIVING MECHANISMS BEHIND DIFFERENCES IN BLEACHING AND DISEASE SUSCEPTIBILITY BETWEEN TWO SCLERACTINIAN CORALS, PSEUDODIPLORIA STRIGOSA AND DIPLORIA LABYRINTHIFORMIS A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in BIOLOGY by Zoe A. Pratte 2015 To: Dean Michael R. Heithaus College of Arts and Sciences This dissertation, written by Zoe A. Pratte, and entitled Investigating the Driving Mechanisms Behind Differences in Bleaching and Disease Susceptibility Between Two Scleractinian Corals, Pseudodiploria strigosa and Diploria labyrinthiformis, having been approved in respect to style and intellectual content, is referred to you for judgment.