Recovery of a Mohua (<Em Class="Sciname">Mohoua
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

New Zealand's Genetic Diversity
1.13 NEW ZEALAND’S GENETIC DIVERSITY NEW ZEALAND’S GENETIC DIVERSITY Dennis P. Gordon National Institute of Water and Atmospheric Research, Private Bag 14901, Kilbirnie, Wellington 6022, New Zealand ABSTRACT: The known genetic diversity represented by the New Zealand biota is reviewed and summarised, largely based on a recently published New Zealand inventory of biodiversity. All kingdoms and eukaryote phyla are covered, updated to refl ect the latest phylogenetic view of Eukaryota. The total known biota comprises a nominal 57 406 species (c. 48 640 described). Subtraction of the 4889 naturalised-alien species gives a biota of 52 517 native species. A minimum (the status of a number of the unnamed species is uncertain) of 27 380 (52%) of these species are endemic (cf. 26% for Fungi, 38% for all marine species, 46% for marine Animalia, 68% for all Animalia, 78% for vascular plants and 91% for terrestrial Animalia). In passing, examples are given both of the roles of the major taxa in providing ecosystem services and of the use of genetic resources in the New Zealand economy. Key words: Animalia, Chromista, freshwater, Fungi, genetic diversity, marine, New Zealand, Prokaryota, Protozoa, terrestrial. INTRODUCTION Article 10b of the CBD calls for signatories to ‘Adopt The original brief for this chapter was to review New Zealand’s measures relating to the use of biological resources [i.e. genetic genetic resources. The OECD defi nition of genetic resources resources] to avoid or minimize adverse impacts on biological is ‘genetic material of plants, animals or micro-organisms of diversity [e.g. genetic diversity]’ (my parentheses). -

Disaggregation of Bird Families Listed on Cms Appendix Ii
Convention on the Conservation of Migratory Species of Wild Animals 2nd Meeting of the Sessional Committee of the CMS Scientific Council (ScC-SC2) Bonn, Germany, 10 – 14 July 2017 UNEP/CMS/ScC-SC2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II (Prepared by the Appointed Councillors for Birds) Summary: The first meeting of the Sessional Committee of the Scientific Council identified the adoption of a new standard reference for avian taxonomy as an opportunity to disaggregate the higher-level taxa listed on Appendix II and to identify those that are considered to be migratory species and that have an unfavourable conservation status. The current paper presents an initial analysis of the higher-level disaggregation using the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World Volumes 1 and 2 taxonomy, and identifies the challenges in completing the analysis to identify all of the migratory species and the corresponding Range States. The document has been prepared by the COP Appointed Scientific Councilors for Birds. This is a supplementary paper to COP document UNEP/CMS/COP12/Doc.25.3 on Taxonomy and Nomenclature UNEP/CMS/ScC-Sc2/Inf.3 DISAGGREGATION OF BIRD FAMILIES LISTED ON CMS APPENDIX II 1. Through Resolution 11.19, the Conference of Parties adopted as the standard reference for bird taxonomy and nomenclature for Non-Passerine species the Handbook of the Birds of the World/BirdLife International Illustrated Checklist of the Birds of the World, Volume 1: Non-Passerines, by Josep del Hoyo and Nigel J. Collar (2014); 2. -

<Em Class="Sciname">Mohoua Albicilla</Em>
Short Note 235 Moore, J.L. 1999. Norfolk Island bird notes, 1977 to 1997. Smart, J.B. 1973. Notes on the occurrence of waders in Notornis 46: 354-364. Fiji. Notornis 18: 267-279. Oliver, W.R.B. 1951. New Zealand birds. 2nd ed. Turbott, E.G. (convener). 1990. Checklist of the birds of New Wellington, A.H. & A.W. Reed. Zealand and the Ross Dependency, Antarctica. Auckland, Pierce, R.J. 1999. Regional patterns of migration in the Random Century New Zealand LtaJ. banded dotterel (Charadrius bicinctus bicinctus). Watling, D. 2001. Aguide to the birds of Fiji B western Polynesia. Notornis 46: 101-122. Suva, Fiji, Environmental Consu1tanl:s (Fiji) Ltd. Skinner, N.J. 1983. The occurrence of waders at Suva Keywords banded dotterel, Churadrius bicinctus, Point, Fiji. Notornis 30: 227-232. Vanuatu. Notornis, 2003, Vol. 50: 235 0029-4470 O The Ornithological Society of New Zealand, Inc. 2003 SHORT NOTE A longevity record for whitehead Mohoua albicilla, Pachycephalidae I. SOUTHEY 640 Waiuku Rd., R.D.3, Pukekohe, New Zealand. B.J. GILL Auckland Museum, Private Bag 92018, Auckland, New Zealand. On 22 January 2001, IS saw a colour-banded white- (1) Male B-41125, red/metal, red/white, banded as head (Mohoua albicilla) in the south-west of Little an adult in January 1985. Barrier Island near the main landing site. It was on (2) Male B-41131, red/metal, red/yellow, banded the flats, about 300 m east of the "Bunkhouse" and as a juvenile in March 1985. about 80 m from the shore. It had a metal band (M) The second bird would not have hatched after and 3 colour-bands in the combination red/metal January 1985. -

Best Practice Techniques for the Translocation of Whiteheads (Popokatea, Mohoua Albicilla)
Best practice techniques for the translocation of whiteheads (popokatea, Mohoua albicilla) Ralph Powlesland and Kevin Parker Cover: Whitehead, Tiritiri Matangi Island. Photo: Martin Sanders. © Copyright April 2014, New Zealand Department of Conservation Published by the Terrestrial Ecosystems Unit, National Office, Science and Capability Group, Department of Conservation, PO Box 10420, The Terrace, Wellington 6143, New Zealand. Editing and design by the Publishing Team, National Office, Department of Conservation, PO Box 10420, The Terrace, Wellington 6143, New Zealand. CONTENTS Abstract 1 1. Introduction 2 2. Animal welfare requirements 3 3. Transfer team 3 4. Time of year for transfer 3 5. Number of transfers 4 6. Composition of transfer group 4 7. Sexing whiteheads 4 7.1 Appearance 4 7.2 Measurements 5 7.3 DNA sexing 6 8. Ageing whiteheads 7 9. Capture 7 10. Transfer to base for ‘processing’ 7 11. Processing the birds 8 12. Temporary housing in aviaries 10 12.1 Capture in the aviary on transfer day 12 13. Feeding 14 14. Whitehead husbandry 15 15. Transfer box design 15 16. Transport 16 17. Release 17 18. Post-release monitoring 17 18.1 Purpose 17 18.2 Recommended monitoring 19 19. Record keeping 19 20. References 21 Appendix 1 Details of report contributors 23 Appendix 2 Feeding protocol for whiteheads being held in temporary aviaries 24 Appendix 3 Recipes for whitehead foods 25 Best practice techniques for the translocation of whiteheads (popokatea, Mohoua albicilla) Ralph Powlesland1 and Kevin Parker2 1 606 Manaroa Road, Manaroa, RD 2, Picton, New Zealand [email protected] 2 Parker Conservation, Auckland, New Zealand parkerconservation.co.nz Abstract This document outlines best practice techniques for the translocation of whiteheads (popokatea, Mohoua albicilla). -

New Zealand Passerines Help Clarify the Diversification of Major Songbird Lineages During the Oligocene
GBE New Zealand Passerines Help Clarify the Diversification of Major Songbird Lineages during the Oligocene Gillian C. Gibb1,*,y, Ryan England2,4,y, Gerrit Hartig2,5, Patricia A. (Trish) McLenachan2, Briar L. Taylor Smith1, Bennet J. McComish2,6, Alan Cooper3, and David Penny2 1Ecology Group, Institute of Agriculture and Environment, Massey University, Palmerston North, New Zealand 2Institute of Fundamental Sciences, Massey University, Palmerston North, New Zealand 3Australian Centre for Ancient DNA, School of Earth and Environmental Sciences, University of Adelaide, South Australia, Australia 4Present address: Forensic Business Group, Institute of Environmental Science and Research (ESR Ltd.), Mt Albert Science Centre, Auckland, New Zealand 5Present address: Starlims Germany GmbH An Abbott Company, Witten, Germany 6Present address: School of Physical Sciences, University of Tasmania, Hobart, Australia *Corresponding author: E-mail: [email protected]. yThese authors contributed equally to this work. Accepted: October 7, 2015 Data deposition: This project has been deposited at GenBank under the accession numbers KC545397-KC545409, KT894672. Abstract Passerines are the largest avian order, and the 6,000 species comprise more than half of all extant bird species. This successful radiation probably had its origin in the Australasian region, but dating this origin has been difficult due to a scarce fossil record and poor biogeographic assumptions. Many of New Zealand’s endemic passerines fall within the deeper branches of the passerine radiation, and a well resolved phylogeny for the modern New Zealand element in the deeper branches of the oscine lineage will help us understand both oscine and passerine biogeography. To this end we present complete mitochondrial genomes representing all families of New Zealand passerines in a phylogenetic framework of over 100 passerine species. -

New Zealand 2018
Field Guides Tour Report New Zealand 2018 Nov 11, 2018 to Nov 29, 2018 Dan Lane & Mark Ayer For our tour description, itinerary, past triplists, dates, fees, and more, please VISIT OUR TOUR PAGE. New Zealand birds have some of the best names.... For instance, this is the Pipipi, a small songbird endemic to New Zealand. Participant David Woods captured this shot of a curious individual. New Zealand is a country that is like no other. It is “at the end of the world” in some respects, having been isolated for millions of years from the rest of the world. Its flora show its link to other southern land masses with such native species as Podocarp conifers, Nothofagus beeches, and fuchsias, all shared with Australia and Patagonia, harkening back to the time of Gondwanaland. The native fauna was all but exclusively made up of birds, but thanks to the arrival of humans (particularly Europeans), what is present today is but a shadow of what once was. Nevertheless, we still can see an impressive variety of birds, including six endemic families, and a whole host of native species. Happily, New Zealanders seem to have taken their unique avifauna to heart, and have made great strives to remove the exotic mammalian predators that have been responsible for the extinction or rarity of so many species. As a result, native bird voices once again fill the beech and podocarp forests, and the predator-free offshore islands are havens for communities of native species. Out tour took us from the southern end of the South Island to Stewart Island, and back north again, crossing Foveaux Strait, along the length of the South Island (and both sides!) to the Cook Strait, and then north across the North Island to Auckland. -

MOHUA) YELLOWHEAD RECOVERY PLAN (Mohoua Ochrocephala
THREATENED SPECIES RECOVERY PLAN SERIES NO.6 (MOHUA) YELLOWHEAD RECOVERY PLAN (Mohoua ochrocephala) Prepared by Colin O'Donnell (Science & Research Division, Christchurch) for the Threatened Species Unit Threatened Species Unit Department of Conservation P.O. Box 10-420 Wellington New Zealand June 1993 ISSN 1170-3806 ISBN 0-478-01483-5 Key words: Yellowhead, mohua, Mohoua ochrocephala, recovery plan, forest birds Frontispiece: Mohoua ochrocephala. Photo: M. Soper CONTENTS The Mohua (or yellowhead, Mohoua ochrocephala) is a small, insectivorous, forest passerine bird, endemic to the South Island. It belongs to an endemic genus along with the whitehead (M. albicilla), and the brown creeper (M. novaezelandiae). All three species have suffered through habitat loss at least since the arrival of Europeans in New Zealand, but unlike the whitehead and brown creeper, the mohua has disappeared from large, relatively unmodified forests and is continuing to decline. Last century mohua were one of the most abundant and conspicuous forest birds in the South Island. Historical records show that they were once present in most forest habitats of the South Island and Stewart Island (some 6.5 million ha). They are now all but absent from 75 % of their former range and much reduction in range has occurred in the last 20 years (O'Donnell & Dilks 1983, Gaze 1985). As a response to concerns about the status of mohua, a workshop was held in 1985 which reviewed the decline, current knowledge, and future research and management possibilities for the species (O'Donnell 1985). As a result of this workshop a monitoring programme was set up at key sites around the South Island (O'Donnell 1986). -

2010 Conference Abstracts
Re-examination of the epic transoceanic migration of the long-tailed cuckoo Eudynamys taitensis (Aves: Cuculidae) Brian J Gill, Auckland Museum, Auckland, New Zealand ([email protected]) Long-tailed cuckoos (Eudynamys taitensis; 125 g) breed only in New Zealand, parasitising three species of Mohoua (Pachycephalidae). After performing perhaps the most remarkable overwater migration of any land bird, they winter in a vast arc of Pacific islands extending 10,000 km from Palau (134.5°E) to Henderson Island (Pitcairn group; 128.3°W). Such an epic migration by so small a bird was originally doubted. After systematic collecting of birds on south Pacific islands by the Whitney South Sea Expedition (1920-32), a 1937 paper by Bogert established the bare details of the migration. This study aims to reassemble data on the long-tailed cuckoo’s migration, using specimens and literature records. The sexes are alike, but immatures (spotted back, rufous underparts) are readily distinguishable from adults (barred back, white underparts), allowing new analysis of migration patterns in relation to age. Preliminary results show that many birds in the wintering grounds have intermediate plumage (are presumably moulting from immatures to adults). A juvenile plumage, not previously noted, has also been identified. At the start of the breeding season (October- December) practically all birds in New Zealand are adults, and immatures in museum collections are overwhelmingly restricted to late summer and autumn. This establishes that all immatures in New Zealand are young-of-the-year. One of the three hosts (yellowhead M. ochrocephala) is now critically endangered. This must mean that the populations of cuckoos adapted to parasitising yellowheads are endangered or extinct now, in proportion to the decline of their host. -

Download Article As 589.6 KB PDF File
6 AvailableNew on-lineZealand at: Journal http://www.newzealandecology.org/nzje/ of Ecology, Vol. 34, No. 1, 2010 special issue: Feathers to Fur The ecological transformation of Aotearoa/New Zealand The origin and history of New Zealand’s terrestrial vertebrates Alan J.D. Tennyson Museum of New Zealand Te Papa Tongarewa, PO Box 467, Wellington, New Zealand (Email: [email protected]) Published on-line: 4 November 2009 Abstract: Since the 1980s, morphological and molecular research has resulted in significant advances in understanding the relationships and origins of the recent terrestrial vertebrate fauna in the New Zealand biogeographic region. This research has led to many taxonomic changes, with a significant increase in the number of bird and reptile species recognised. It has also resulted in the recognition of several more Holocene (<10 000 years ago) bird species extinctions. The conclusion that Holocene extinctions were primarily caused by human- hunting and predation by other introduced mammals (particularly rats and cats) has been supported by new data. Despite many local eradications of introduced pests, the number of introduced species has increased, with the establishment of five more foreign birds and (on Norfolk Island) the house gecko (Hemidactylus frenatus). Many new, significant New Zealand vertebrate fossils have been reported, including more dinosaurs from the Cretaceous, and the first Tertiary records of frogs, rhynchocephalids, lizards, crocodylians, bats and a terrestrial “Mesozoic ghost” mammal from the Early Miocene near St Bathans. For birds, the earliest known penguins in the world have been discovered, and there are intriguing Late Cretaceous – Early Paleocene remains still awaiting detailed description. -

New Zealand Passerines Help Clarify the Diversification of Major Songbird Lineages During the Oligocene
GBE New Zealand Passerines Help Clarify the Diversification of Major Songbird Lineages during the Oligocene Gillian C. Gibb1,*,y, Ryan England2,4,y, Gerrit Hartig2,5, Patricia A. (Trish) McLenachan2, Briar L. Taylor Smith1, Bennet J. McComish2,6, Alan Cooper3, and David Penny2 1Ecology Group, Institute of Agriculture and Environment, Massey University, Palmerston North, New Zealand 2 Institute of Fundamental Sciences, Massey University, Palmerston North, New Zealand Downloaded from https://academic.oup.com/gbe/article-abstract/7/11/2983/2939550 by guest on 12 December 2018 3Australian Centre for Ancient DNA, School of Earth and Environmental Sciences, University of Adelaide, South Australia, Australia 4Present address: Forensic Business Group, Institute of Environmental Science and Research (ESR Ltd.), Mt Albert Science Centre, Auckland, New Zealand 5Present address: Starlims Germany GmbH An Abbott Company, Witten, Germany 6Present address: School of Physical Sciences, University of Tasmania, Hobart, Australia *Corresponding author: E-mail: [email protected]. yThese authors contributed equally to this work. Accepted: October 7, 2015 Data deposition: This project has been deposited at GenBank under the accession numbers KC545397-KC545409, KT894672. Abstract Passerines are the largest avian order, and the 6,000 species comprise more than half of all extant bird species. This successful radiation probably had its origin in the Australasian region, but dating this origin has been difficult due to a scarce fossil record and poor biogeographic assumptions. Many of New Zealand’s endemic passerines fall within the deeper branches of the passerine radiation, and a well resolved phylogeny for the modern New Zealand element in the deeper branches of the oscine lineage will help us understand both oscine and passerine biogeography. -

Or POLYMYODI): Oscines (Songbirds
Text extracted from Gill B.J.; Bell, B.D.; Chambers, G.K.; Medway, D.G.; Palma, R.L.; Scofield, R.P.; Tennyson, A.J.D.; Worthy, T.H. 2010. Checklist of the birds of New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency, Antarctica. 4th edition. Wellington, Te Papa Press and Ornithological Society of New Zealand. Pages 275, 279-280 & 293-295. Order PASSERIFORMES: Passerine (Perching) Birds See Christidis & Boles (2008) for a review of recent studies relevant to the higher-level systematics of the passerine birds. Suborder PASSERES (or POLYMYODI): Oscines (Songbirds) The arrangement of songbirds in the 1970 Checklist (Checklist Committee 1970) was based on the premise that the species endemic to the Australasian region were derived directly from Eurasian groups and belonged in Old World families (e.g. Gerygone and Petroica in Muscicapidae). The 1990 Checklist (Checklist Committee 1990) followed the Australian lead in allocating various native songbirds to their own Australasian families (e.g. Gerygone to Acanthizidae, and Petroica to Eopsaltriidae), but the sequence was still based largely on the old Peters-Mayr arrangement. Since the late 1980s, when the 1990 Checklist was finalised, evidence from molecular biology, especially DNA studies, has shown that most of the Australian and New Zealand endemic songbirds are the product of a major Australasian radiation parallel to the radiation of songbirds in Eurasia and elsewhere. Many superficial morphological and ecological similarities between Australasian and Eurasian songbirds are the result of convergent evolution. Sibley & Ahlquist (1985, 1990) and Sibley et al. (1988) recognised a division of the songbirds into two groups which were called Corvida and Passerida (Sibley & Ahlquist 1990). -
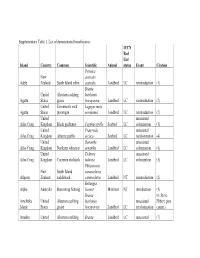
Supplementary Table 1. List of Demonstrated Beneficiaries
Supplementary Table 1. List of demonstrated beneficiaries. IUCN Red List Island Country Common Scientific Animal status Event Citation Petroica New australis Adele Zealand South Island robin australis Landbird LC reintroduction (1) Branta United Aleutian cackling hutchinsii Agattu States goose leucopareia Landbird LC reintroduction (2) United Evermann's rock Lagopus muta Agattu States ptarmigan evermanni Landbird LC reintroduction (2) United unassisted Ailsa Craig Kingdom Black guillemot Cepphus grylle Seabird LC colonization (3) United Fratercula unassisted Ailsa Craig Kingdom Atlantic puffin arctica Seabird LC recolonization (4) United Oenanthe unassisted Ailsa Craig Kingdom Northern wheatear oenanthe Landbird LC colonization (4) United Tadorna unassisted Ailsa Craig Kingdom Common shelduck tadorna Landbird LC colonization (3) Philesturnus New South Island carunculatus Allports Zealand saddleback carunculatus Landbird NT reintroduction (2) Bettongia Alpha Australia Burrowing bettong lesueur Mammal NT introduction (5) Branta (6; Steve Amchitka United Aleutian cackling hutchinsii unassisted Ebbert, pers. Island States goose leucopareia Landbird LC recolonization comm.) Amukta United Aleutian cackling Branta Landbird LC unassisted (7) IUCN Red List Island Country Common Scientific Animal status Event Citation States goose hutchinsii recolonization leucopareia Sally Amy Poncet, Island/Outer United Cinclodes unassisted unpublished Knob Kingdom Tussacbird antarcticus Landbird LC recolonization data Sally Amy Poncet, Island/Outer United unpublished