Emotional and Spatial Learning in Goldfish Is Dependent on Different
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fish Do Not Feel Pain and Its Implications for Understanding Phenomenal Consciousness
Biol Philos (2015) 30:149–165 DOI 10.1007/s10539-014-9469-4 Fish do not feel pain and its implications for understanding phenomenal consciousness Brian Key Received: 14 April 2014 / Accepted: 6 December 2014 / Published online: 16 December 2014 Ó The Author(s) 2014. This article is published with open access at Springerlink.com Abstract Phenomenal consciousness or the subjective experience of feeling sen- sory stimuli is fundamental to human existence. Because of the ubiquity of their subjective experiences, humans seem to readily accept the anthropomorphic extension of these mental states to other animals. Humans will typically extrapolate feelings of pain to animals if they respond physiologically and behaviourally to noxious stimuli. The alternative view that fish instead respond to noxious stimuli reflexly and with a limited behavioural repertoire is defended within the context of our current understanding of the neuroanatomy and neurophysiology of mental states. Consequently, a set of fundamental properties of neural tissue necessary for feeling pain or experiencing affective states in vertebrates is proposed. While mammals and birds possess the prerequisite neural architecture for phenomenal consciousness, it is concluded that fish lack these essential characteristics and hence do not feel pain. Keywords Fish Á Pain Á Phenomenal consciousness Á Affective states Á Avoidance learning Á Neocortex Á Pallium Introduction There is a belief in some scientific and lay communities that because fish respond behaviourally to noxious stimuli, then ipso facto, fish feel pain. Sneddon (2011) clearly articulates the logic by stating: ‘‘to explore the possibility of pain perception in nonhumans we use indirect measures similar to those used for human infants who cannot convey whether they are in pain. -

Discourse and Identity: Representations of Women in Little Mix’S Songs
DISCOURSE AND IDENTITY: REPRESENTATIONS OF WOMEN IN LITTLE MIX’S SONGS BY ALYA AISYAH BINTI AMRAN INTERNATIONAL ISLAMIC UNIVERSITY MALAYSIA 2020 DISCOURSE AND IDENTITY: REPRESENTATIONS OF WOMEN IN LITTLE MIX’S SONGS BY ALYA AISYAH BINTI AMRAN A Final Year Project submitted in fulfilment of the requirement for the degree of English for International Communications Kulliyyah of Languages and Management International Islamic University Malaysia JANUARY 2020 ABSTRACT Discrimination against women is a global issue and has been for years. Even in developing countries women experience biasness on the basis of their gender. The study aims to identify the different social roles and traits of women that can be found in song lyrics. Past studies explored various issues of what women face in their daily lives whether it be in education, social relationships and decision making in context of gender discrimination that are present in the media and songs. This is an exploratory study on representations of women in songs. The artist chosen for this study is the United Kingdom’s all female group, Little Mix. A qualitative research approach was used to fulfill the objectives of this research. A total of 29 Little Mix songs were chosen as the data and Membership Categorization Analysis was used as the framework of this research. Based on the analyses, a total of five social roles and three traits were found shared among women that were present in the songs. Women were represented both positively and negatively in Little Mix’s songs. The study may contribute to future studies that uses Membership Categorization Analysis as their framework of study. -

The Structural Model: a Theory Linking Connections, Plasticity, Pathology, Development and Evolution of the Cerebral Cortex
Brain Structure and Function https://doi.org/10.1007/s00429-019-01841-9 REVIEW The Structural Model: a theory linking connections, plasticity, pathology, development and evolution of the cerebral cortex Miguel Ángel García‑Cabezas1 · Basilis Zikopoulos2,3 · Helen Barbas1,3 Received: 11 October 2018 / Accepted: 29 January 2019 © Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract The classical theory of cortical systematic variation has been independently described in reptiles, monotremes, marsupials and placental mammals, including primates, suggesting a common bauplan in the evolution of the cortex. The Structural Model is based on the systematic variation of the cortex and is a platform for advancing testable hypotheses about cortical organization and function across species, including humans. The Structural Model captures the overall laminar structure of areas by dividing the cortical architectonic continuum into discrete categories (cortical types), which can be used to test hypotheses about cortical organization. By type, the phylogenetically ancient limbic cortices—which form a ring at the base of the cerebral hemisphere—are agranular if they lack layer IV, or dysgranular if they have an incipient granular layer IV. Beyond the dysgranular areas, eulaminate type cortices have six layers. The number and laminar elaboration of eulaminate areas differ depending on species or cortical system within a species. The construct of cortical type retains the topology of the systematic variation of the cortex and forms the basis for a predictive Structural Model, which has successfully linked cortical variation to the laminar pattern and strength of cortical connections, the continuum of plasticity and stability of areas, the regularities in the distribution of classical and novel markers, and the preferential vulnerability of limbic areas to neurodegenerative and psychiatric diseases. -

Perspectives
PERSPECTIVES reptiles, to birds and mammals, to primates OPINION and, finally, to humans — ascending from ‘lower’ to ‘higher’ intelligence in a chrono- logical series. They believed that the brains Avian brains and a new understanding of extant vertebrates retained ancestral structures, and, therefore, that the origin of of vertebrate brain evolution specific human brain subdivisions could be traced back in time by examining the brains of extant non-human vertebrates. In The Avian Brain Nomenclature Consortium* making such comparisons, they noted that the main divisions of the human CNS — Abstract | We believe that names have a pallium is nuclear, and the mammalian the spinal cord, hindbrain, midbrain, thala- powerful influence on the experiments we cortex is laminar in organization, the avian mus, cerebellum and cerebrum or telen- do and the way in which we think. For this pallium supports cognitive abilities similar cephalon — were present in all vertebrates reason, and in the light of new evidence to, and for some species more advanced than, (FIG. 1a). Edinger, however, noted that the about the function and evolution of the those of many mammals. To eliminate these internal organization of the telencephala vertebrate brain, an international consortium misconceptions, an international forum of showed the most pronounced differences of neuroscientists has reconsidered the neuroscientists (BOX 1) has, for the first time between species. In mammals, the outer traditional, 100-year-old terminology that is in 100 years, developed new terminology that part of the telencephalon was found to have used to describe the avian cerebrum. Our more accurately reflects our current under- prominently layered grey matter (FIG. -

Foxg1confines Cajal–Retzius Neuronogenesis and Hippocampal
The Journal of Neuroscience, April 27, 2005 • 25(17):4435–4441 • 4435 Development/Plasticity/Repair Foxg1 Confines Cajal–Retzius Neuronogenesis and Hippocampal Morphogenesis to the Dorsomedial Pallium Luca Muzio and Antonello Mallamaci Department of Biological and Technological Research, San Raffaele Scientific Institute, 20132 Milan, Italy It has been suggested that cerebral cortex arealization relies on positional values imparted to early cortical neuroblasts by transcription factor genes expressed within the pallial field in graded ways. Foxg1, encoding for one of these factors, previously was reported to be necessary for basal ganglia morphogenesis, proper tuning of cortical neuronal differentiation rates, and the switching of cortical neuro- blasts from early generation of primordial plexiform layer to late production of cortical plate. Being expressed along a rostral/lateral high- to-caudal/medial low gradient, Foxg1, moreover, could contribute to shaping the cortical areal profile as a repressor of caudomedial fates. Wetestedthispredictionbyavarietyofapproachesandfoundthatitwascorrect.WefoundthatoverproductionofCajal–Retziusneurons characterizing Foxg1Ϫ/Ϫ mutants does not arise specifically from blockage of laminar histogenetic progression of neocortical neuro- blasts, as reported previously, but rather reflects lateral-to-medial repatterning of their cortical primordium. Even if lacking a neocortical plate, Foxg1Ϫ/Ϫ embryos give rise to structures, which, for molecular properties and birthdating profile, are highly reminiscent of hippocampal plate and dentate blade. Remarkably, in the absence of Foxg1, additional inactivation of the medial fates promoter Emx2, although not suppressing cortical specification, conversely rescues overproduction of Reelin on neurons. Key words: Foxg1; Emx2; Wnt types; hippocampus; neocortex; Cajal–Retzius cells Introduction neuronogenesis (Xuan et al., 1995; Dou et al., 1999; Seoane et al., Areal specification of cortical neurons is an extremely complex 2004). -

{DOWNLOAD} Viva Coldplay: a Biography Ebook Free Download
VIVA COLDPLAY: A BIOGRAPHY PDF, EPUB, EBOOK Martin Roach | 240 pages | 01 Nov 2010 | OMNIBUS PRESS | 9781849385466 | English | London, United Kingdom Viva Coldplay: A Biography PDF Book CBC News. In the United States, the song was released as the lead single from the then-untitled debut album. Books Video icon An illustration of two cells of a film strip. The Telegraph. Archived from the original on 28 August Official Charts. Retrieved 7 September Archived from the original on 15 May Video Audio icon An illustration of an audio speaker. Retrieved 24 April Quotes from Coldplay: Viva Co After completing their final examinations, Coldplay signed a five-album contract with Parlophone in early Archived from the original on 7 July Retrieved 10 May Retrieved 6 February Retrieved 28 September They decided to relocate in Liverpool, where they recorded some of the songs on Parachutes. The band played beneath Hope , a giant year-old skeleton of a blue whale in the museum's great hall. Home 1 Books 2. Chris Martin is lead singer, guitarist and pianist for the band Coldplay. Their performance included a duet with Barry Gibb , the last surviving member of the Bee Gees. There are no discussion topics on this book yet. Sort order. Retrieved 2 October English explorer Martin Frobisher is best known for his attempts to discover a Northwest Passage and his voyages to Labrador and Frobisher Bay in Canada. In , it was unusual for a pop group to have a monthly magazine devoted exclusively to their career. Viva Coldplay: A Biography Writer Retrieved 12 December Archived from the original on 8 July Archived from the original on 24 January Together, they debuted the song live at the Brit Awards with Chris Martin also performing a tribute song to the late George Michael. -

TREASURES O F DARKNESS
TREASURES of DARKNESS Copyright © 2014, Jane Johnson Photography & Design, LLC All rights reserved. ISBN-13: 978-0692258187 • ISBN-10: 0692258183 Unless otherwise stated, all Scripture citations are from the NKJV © 1994 by Thomas Nelson, Inc. Cover design by Jane Johnson Design Cover photograph by Jenna Michelle Photography HELLO & WELCOME Thank you so much for being here. For embarking on a brand new journey with me. For being brave and open and hungering for more of Him. The words on these pages are the result of a dish cloth of faith being wrung dry. They are straight out of my prayer journal. Real. Raw. Honest. More honest and vulnerable than I have been outside of conversations with Him ... ever. Which is scary for me. But glory for Him. I wrote this study in 2011. It's nine weeks long, with five days of homework for each week. A God-breathed, Holy-Spirit-inspired, I-could-never-have-done-this-if-it-wasn't- for-Him type of study. We were five years into our wait for a family. My best friend was five months into an 18-month battle with stage four cancer - a battle she lost one year later. I was neck-deep in heartache. Words rang hollow in my heart pierced by pain.1 But through the plucking of my heartstrings came this. The book you're holding in your hands. Cover uncreased. Pages fresh. Treasures undiscovered. It's such an honor to have you here with me. Truly. Praying His Spirit fills you with every word, 1 Ray Stedman, Let God Be God SMALL STEPS Generally, a book's forward is written by a published author or person of significance. -

Proper 19 (Series B) “Praise the One Who Breaks the Darkness”
Proper 19 (Series B) “Praise the One Who Breaks the Darkness” (Lutheran Service Book #849) For our hymn of the day assigned to this Sunday, we are fortunate to have insight from the author himself, Rev. Rusty Edwards. He says about his hymn text: “Have you ever started to do one thing, and then ended up doing something else? That’s exactly what happened with the writing of ‘Praise the One Who Breaks the Darkness.’ I intended to create a Bible study. This study would look at the life and unique ministry of Jesus. I wanted people to get closer to Jesus. We would ask the question, ‘What on earth did Jesus really do?’ Of course, the way to build a study such as this was to go back to the Bible, and read again, with new eyes, the story of his life. I did just that, and was amazed at what he did. I began with the gospel of Mark, but then continued with the three other gospels. As I prayed, studied and thanked God for the life of Jesus, I began to write down some of his works. The list grew longer. Suddenly, I gazed down at the list, and the list looked almost like a hymn. That’s when the Bible study got put on hold for a while! I wish I could take credit for the wide use of the hymn, but I really can’t. The Holy Spirit was the one who changed my mind, the Bible study became a song. The popularity of this song has everything to do with the message of the story. -
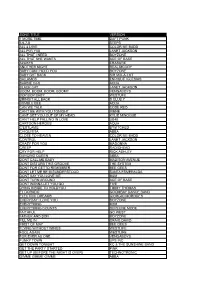
Song Title Version 1 More Time Daft Punk 5,6,7,8 Steps All 4 L0ve Color Me Badd All F0r Y0u Janet Jackson All That I Need Boyzon
SONG TITLE VERSION 1 MORE TIME DAFT PUNK 5,6,7,8 STEPS ALL 4 L0VE COLOR ME BADD ALL F0R Y0U JANET JACKSON ALL THAT I NEED BOYZONE ALL THAT SHE WANTS ACE OF BASE ALWAYS ERASURE ANOTHER NIGHT REAL MCCOY BABY CAN I HOLD YOU BOYZONE BABY G0T BACK SIR MIX-A-LOT BAILAMOS ENRIQUE IGLESIAS BARBIE GIRL AQUA BLACK CAT JANET JACKSON BOOM, BOOM, BOOM, BOOM!! VENGABOYS BOP BOP BABY WESTUFE BRINGIT ALL BACK S CLUB 7 BUMBLE BEE AQUA CAN WE TALK CODE RED CAN'T BE WITH YOU TONIGHT IRENE CANT GET YOU OUT OF MY HEAD KYLIE MINOGUE CAN'T HELP FALLING IN LOVE UB40 CARTOON HEROES AQUA C'ESTLAVIE B*WITCHED CHIQUITITA ABBA CLOSE TO HEAVEN COLOR ME BADD CONTROL JANET JACKSON CRAZY FOR YOU MADONNA CREEP RADIOHEAD CRY FOR HELP RICK ASHLEY DANCING QUEEN ABBA DONT CALL ME BABY MADISON AVENUE DONT DISTURB THIS GROOVE THE SYSTEM DONT FOR GETTO REMEMBER BEE GEES DONT LET ME BE MISUNDERSTOOD SANTA ESMERALDA DONT SAY YOU LOVE ME M2M DONT TURN AROUND ACE OF BASE DONT WANNA LET YOU GO FIVE DYING INSIDE TO HOLDYOU TIMMY THOMAS EL DORADO GOOMBAY DANCE BAND ELECTRIC DREAMS GIORGIO MORODES EVERYDAY I LOVE YOU BOYZONE EVERYTHING M2M EVERYTHING COUNTS DEPECHE MODE FAITHFUL GO WEST FATHER AND SON BOYZONE FILL ME IN CRAIG DAVID FIRST OF MAY BEE GEES FLYING WITHOUT WINGS WESTLIFE FOOL AGAIN WESTLIFE FOR EVER AS ONE VENGABOYS FUNKY TOWN UPS INC GET DOWN TONIGHT KC & THE SUNSHINE BAND GET THE PARTY STARTED PINK GET UP (BEFORE THE NIGHT IS OVER) TECHNOTRONIC GIMME GIMME GIMME ABBA HAPPY SONG BONEY M. -

Broom Fish Brains Pain
Pre-publication copy Broom, D.M. 2016. Fish brains and behaviour indicate capacity for feeling pain. Animal Sentience, 2016.010 (5 pages). Fish brains, as well as fish behaviour, indicate capacity for awareness and feeling pain Donald M. Broom Centre for Anthrozoology and Animal Welfare Department of Veterinary Medicine University of Cambridge Madingley Road Cambridge CB3 0ES U.K. [email protected] http://www.neuroscience.cam.ac.uk/directory/profile.php?dmb16 Keywords pain sentience welfare fish feelings emotions brain behaviour Abstract Studies of behaviour are of major importance in understanding human pain and pain in other animals such as fish. Almost all of the characteristics of the mammalian pain system are also described for fish. Emotions, feelings and learning from these are controlled in the fish brain in areas anatomically different but functionally very similar to those in mammals. The evidence of pain and fear system function in fish is so similar to that in humans and other mammals that it is logical to conclude that fish feel fear and pain. Fish are sentient beings. Key (2015) is scornful about evidence from studies of fish behaviour indicating that fish are aware and feel pain but presents a thorough explanation of the pain system in the human brain and concludes that fish could not feel pain, or have any other feelings, as they do not have the brain structures that allow pain and other feelings in humans. Section 2 of his paper emphasises “the cortical origins of human pain” and states that “structure determines function”, eXplaining the functions of the five layers of the human cortex. -

The Evolutionary Development of the Brain As It Pertains to Neurosurgery
Open Access Original Article DOI: 10.7759/cureus.6748 The Evolutionary Development of the Brain As It Pertains to Neurosurgery Jaafar Basma 1 , Natalie Guley 2 , L. Madison Michael II 3 , Kenan Arnautovic 3 , Frederick Boop 3 , Jeff Sorenson 3 1. Neurological Surgery, University of Tennessee Health Science Center, Memphis, USA 2. Neurological Surgery, University of Arkansas for Medical Sciences, Little Rock, USA 3. Neurological Surgery, Semmes-Murphey Clinic, Memphis, USA Corresponding author: Jaafar Basma, [email protected] Abstract Background Neuroanatomists have long been fascinated by the complex topographic organization of the cerebrum. We examined historical and modern phylogenetic theories pertaining to microneurosurgical anatomy and intrinsic brain tumor development. Methods Literature and history related to the study of anatomy, evolution, and tumor predilection of the limbic and paralimbic regions were reviewed. We used vertebrate histological cross-sections, photographs from Albert Rhoton Jr.’s dissections, and original drawings to demonstrate the utility of evolutionary temporal causality in understanding anatomy. Results Phylogenetic neuroanatomy progressed from the substantial works of Alcmaeon, Herophilus, Galen, Vesalius, von Baer, Darwin, Felsenstein, Klingler, MacLean, and many others. We identified two major modern evolutionary theories: “triune brain” and topological phylogenetics. While the concept of “triune brain” is speculative and highly debated, it remains the most popular in the current neurosurgical literature. Phylogenetics inspired by mathematical topology utilizes computational, statistical, and embryological data to analyze the temporal transformations leading to three-dimensional topographic anatomy. These transformations have shaped well-defined surgical planes, which can be exploited by the neurosurgeon to access deep cerebral targets. The microsurgical anatomy of the cerebrum and the limbic system is redescribed by incorporating the dimension of temporal causality. -

Sequential Pattern of Sublayer Formation in the Paleocortex and Neocortex
Medical Molecular Morphology (2020) 53:168–176 https://doi.org/10.1007/s00795-020-00245-7 ORIGINAL PAPER Sequential pattern of sublayer formation in the paleocortex and neocortex Makoto Nasu1 · Kenji Shimamura2 · Shigeyuki Esumi1 · Nobuaki Tamamaki1 Received: 17 December 2019 / Accepted: 13 January 2020 / Published online: 30 January 2020 © The Japanese Society for Clinical Molecular Morphology 2020 Abstract The piriform cortex (paleocortex) is the olfactory cortex or the primary cortex for the sense of smell. It receives the olfac- tory input from the mitral and tufted cells of the olfactory bulb and is involved in the processing of information pertaining to odors. The piriform cortex and the adjoining neocortex have diferent cytoarchitectures; while the former has a three-layered structure, the latter has a six-layered structure. The regulatory mechanisms underlying the building of the six-layered neo- cortex are well established; in contrast, less is known about of the regulatory mechanisms responsible for structure formation of the piriform cortex. The diferences as well as similarities in the regulatory mechanisms between the neocortex and the piriform cortex remain unclear. Here, the expression of neocortical layer-specifc genes in the piriform cortex was examined. Two sublayers were found to be distinguished in layer II of the piriform cortex using Ctip2/Bcl11b and Brn1/Pou3f3. The sequential expression pattern of Ctip2 and Brn1 in the piriform cortex was similar to that detected in the neocortex, although the laminar arrangement in the piriform cortex exhibited an outside-in arrangement, unlike that observed in the neocortex. Keywords Piriform cortex (paleocortex) · Sublayer · Sequential expression · Ctip2/Bcl11b · Brn1/Pou3f3 Introduction six-layered cortex, which is a mammal-specifc feature, are well established; sequential expression of transcription fac- The piriform cortex (paleocortex) is the olfactory cortex or tors is involved in determining cell identities, and late-born the primary cortex for the sense of smell.