Top 5 Elements on the Surface of Mercury
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ices on Mercury: Chemistry of Volatiles in Permanently Cold Areas of Mercury’S North Polar Region
Icarus 281 (2017) 19–31 Contents lists available at ScienceDirect Icarus journal homepage: www.elsevier.com/locate/icarus Ices on Mercury: Chemistry of volatiles in permanently cold areas of Mercury’s north polar region ∗ M.L. Delitsky a, , D.A. Paige b, M.A. Siegler c, E.R. Harju b,f, D. Schriver b, R.E. Johnson d, P. Travnicek e a California Specialty Engineering, Pasadena, CA b Dept of Earth, Planetary and Space Sciences, University of California, Los Angeles, CA c Planetary Science Institute, Tucson, AZ d Dept of Engineering Physics, University of Virginia, Charlottesville, VA e Space Sciences Laboratory, University of California, Berkeley, CA f Pasadena City College, Pasadena, CA a r t i c l e i n f o a b s t r a c t Article history: Observations by the MESSENGER spacecraft during its flyby and orbital observations of Mercury in 2008– Received 3 January 2016 2015 indicated the presence of cold icy materials hiding in permanently-shadowed craters in Mercury’s Revised 29 July 2016 north polar region. These icy condensed volatiles are thought to be composed of water ice and frozen Accepted 2 August 2016 organics that can persist over long geologic timescales and evolve under the influence of the Mercury Available online 4 August 2016 space environment. Polar ices never see solar photons because at such high latitudes, sunlight cannot Keywords: reach over the crater rims. The craters maintain a permanently cold environment for the ices to persist. Mercury surface ices magnetospheres However, the magnetosphere will supply a beam of ions and electrons that can reach the frozen volatiles radiolysis and induce ice chemistry. -

HISTORY of LEAD POISONING in the WORLD Dr. Herbert L. Needleman Introduction the Center for Disease Control Classified the Cause
HISTORY OF LEAD POISONING IN THE WORLD Dr. Herbert L. Needleman Introduction The Center for Disease Control classified the causes of disease and death as follows: 50 % due to unhealthy life styles 25 % due to environment 25% due to innate biology and 25% due to inadequate health care. Lead poisoning is an environmental disease, but it is also a disease of life style. Lead is one of the best-studied toxic substances, and as a result we know more about the adverse health effects of lead than virtually any other chemical. The health problems caused by lead have been well documented over a wide range of exposures on every continent. The advancements in technology have made it possible to research lead exposure down to very low levels approaching the limits of detection. We clearly know how it gets into the body and the harm it causes once it is ingested, and most importantly, how to prevent it! Using advanced technology, we can trace the evolution of lead into our environment and discover the health damage resulting from its exposure. Early History Lead is a normal constituent of the earth’s crust, with trace amounts found naturally in soil, plants, and water. If left undisturbed, lead is practically immobile. However, once mined and transformed into man-made products, which are dispersed throughout the environment, lead becomes highly toxic. Solely as a result of man’s actions, lead has become the most widely scattered toxic metal in the world. Unfortunately for people, lead has a long environmental persistence and never looses its toxic potential, if ingested. -

Bepicolombo - a Mission to Mercury
BEPICOLOMBO - A MISSION TO MERCURY ∗ R. Jehn , J. Schoenmaekers, D. Garc´ıa and P. Ferri European Space Operations Centre, ESA/ESOC, Darmstadt, Germany ABSTRACT BepiColombo is a cornerstone mission of the ESA Science Programme, to be launched towards Mercury in July 2014. After a journey of nearly 6 years two probes, the Magneto- spheric Orbiter (JAXA) and the Planetary Orbiter (ESA) will be separated and injected into their target orbits. The interplanetary trajectory includes flybys at the Earth, Venus (twice) and Mercury (four times), as well as several thrust arcs provided by the solar electric propulsion module. At the end of the transfer a gravitational capture at the weak stability boundary is performed exploiting the Sun gravity. In case of a failure of the orbit insertion burn, the spacecraft will stay for a few revolutions in the weakly captured orbit. The arrival conditions are chosen such that backup orbit insertion manoeuvres can be performed one, four or five orbits later with trajectory correction manoeuvres of less than 15 m/s to compensate the Sun perturbations. Only in case that no manoeuvre can be performed within 64 days (5 orbits) after the nominal orbit insertion the spacecraft will leave Mercury and the mission will be lost. The baseline trajectory has been designed taking into account all operational constraints: 90-day commissioning phase without any thrust; 30-day coast arcs before each flyby (to allow for precise navigation); 7-day coast arcs after each flyby; 60-day coast arc before orbit insertion; Solar aspect angle constraints and minimum flyby altitudes (300 km at Earth and Venus, 200 km at Mercury). -

The Search for Another Earth – Part II
GENERAL ARTICLE The Search for Another Earth – Part II Sujan Sengupta In the first part, we discussed the various methods for the detection of planets outside the solar system known as the exoplanets. In this part, we will describe various kinds of exoplanets. The habitable planets discovered so far and the present status of our search for a habitable planet similar to the Earth will also be discussed. Sujan Sengupta is an 1. Introduction astrophysicist at Indian Institute of Astrophysics, Bengaluru. He works on the The first confirmed exoplanet around a solar type of star, 51 Pe- detection, characterisation 1 gasi b was discovered in 1995 using the radial velocity method. and habitability of extra-solar Subsequently, a large number of exoplanets were discovered by planets and extra-solar this method, and a few were discovered using transit and gravi- moons. tational lensing methods. Ground-based telescopes were used for these discoveries and the search region was confined to about 300 light-years from the Earth. On December 27, 2006, the European Space Agency launched 1The movement of the star a space telescope called CoRoT (Convection, Rotation and plan- towards the observer due to etary Transits) and on March 6, 2009, NASA launched another the gravitational effect of the space telescope called Kepler2 to hunt for exoplanets. Conse- planet. See Sujan Sengupta, The Search for Another Earth, quently, the search extended to about 3000 light-years. Both Resonance, Vol.21, No.7, these telescopes used the transit method in order to detect exo- pp.641–652, 2016. planets. Although Kepler’s field of view was only 105 square de- grees along the Cygnus arm of the Milky Way Galaxy, it detected a whooping 2326 exoplanets out of a total 3493 discovered till 2Kepler Telescope has a pri- date. -

ESTIMATION of FISSION-PRODUCT GAS PRESSURE in URANIUM DIOXIDE CERAMIC FUEL ELEMENTS by Wuzter A
NASA TECHNICAL NOTE NASA TN D-4823 - - .- j (2. -1 "-0 -5 M 0-- N t+=$j oo w- P LOAN COPY: RET rm 3 d z c 4 c/) 4 z ESTIMATION OF FISSION-PRODUCT GAS PRESSURE IN URANIUM DIOXIDE CERAMIC FUEL ELEMENTS by WuZter A. PuuZson una Roy H. Springborn Lewis Reseurcb Center Clevelund, Ohio NATIONAL AERONAUTICS AND SPACE ADMINISTRATION WASHINGTON, D. C. NOVEMBER 1968 i 1 TECH LIBRARY KAFB, NM I 111111 lllll IllH llll lilll1111111111111 Ill1 01317Lb NASA TN D-4823 ESTIMATION OF FISSION-PRODUCT GAS PRESSURE IN URANIUM DIOXIDE CERAMIC FUEL ELEMENTS By Walter A. Paulson and Roy H. Springborn Lewis Research Center Cleveland, Ohio NATIONAL AERONAUTICS AND SPACE ADMINISTRATION For sale by the Clearinghouse for Federal Scientific and Technical Information Springfield, Virginia 22151 - CFSTl price $3.00 ABSTRACl Fission-product gas pressure in macroscopic voids was calculated over the tempera- ture range of 1000 to 2500 K for clad uranium dioxide fuel elements operating in a fast neutron spectrum. The calculated fission-product yields for uranium-233 and uranium- 235 used in the pressure calculations were based on experimental data compiled from various sources. The contributions of cesium, rubidium, and other condensible fission products are included with those of the gases xenon and krypton. At low temperatures, xenon and krypton are the major contributors to the total pressure. At high tempera- tures, however, cesium and rubidium can make a considerable contribution to the total pressure. ii ESTIMATION OF FISSION-PRODUCT GAS PRESSURE IN URANIUM DIOXIDE CERAMIC FUEL ELEMENTS by Walter A. Paulson and Roy H. -

Mercury Education
MERCURY The Slippery, Silent Toxin to Dispose of Carefully You may be surprised to find mercury in your home, business and places you go often. When spilled or not disposed of carefully, it poses a risk to human and ecosystem health. DID YOU KNOW? Mercury is the only metal that is liquid at room Why is Mercury Such a Problem? temperature. Mercury, or quicksilver, is an element that serves many useful 80 purposes – when contained. It conducts electricity, is used in thermometers, thermostats, barometers, blood pressure cuffs and many other everyday items such as some alarm clocks Mercury 200.59 and irons. The most common use is in today’s fluorescent light bulbs that use mercury vapor. Read the reverse The trouble comes when mercury is not disposed of properly for safe ways to or worse, when it spills. Mercury is a toxic element that can dispose of mercury. enter the body through an open wound or by inhaling or ingesting it. It can cause damage to nerves, the liver and kidneys, as well as a number of other symptoms. If mercury is poured down the drain or dumped into the sewer, it seeps into lakes and waterways, a chemical process occurs, converting it to deadly methylmercury, potentially contaminating the fish and animals we eat. When products containing mercury are placed in the trash or poured down the drain, the mercury does not disappear. It ends up in the environment via landfills and wastewater treatment facilities. Now that you know some of the items that contain mercury, you can make Never touch or vacuum up buying decisions that reduce the amount of mercury in your home or office, spilled mercury in any form, and that assure safe disposal of products that contain mercury. -

Mariner to Mercury, Venus and Mars
NASA Facts National Aeronautics and Space Administration Jet Propulsion Laboratory California Institute of Technology Pasadena, CA 91109 Mariner to Mercury, Venus and Mars Between 1962 and late 1973, NASA’s Jet carry a host of scientific instruments. Some of the Propulsion Laboratory designed and built 10 space- instruments, such as cameras, would need to be point- craft named Mariner to explore the inner solar system ed at the target body it was studying. Other instru- -- visiting the planets Venus, Mars and Mercury for ments were non-directional and studied phenomena the first time, and returning to Venus and Mars for such as magnetic fields and charged particles. JPL additional close observations. The final mission in the engineers proposed to make the Mariners “three-axis- series, Mariner 10, flew past Venus before going on to stabilized,” meaning that unlike other space probes encounter Mercury, after which it returned to Mercury they would not spin. for a total of three flybys. The next-to-last, Mariner Each of the Mariner projects was designed to have 9, became the first ever to orbit another planet when two spacecraft launched on separate rockets, in case it rached Mars for about a year of mapping and mea- of difficulties with the nearly untried launch vehicles. surement. Mariner 1, Mariner 3, and Mariner 8 were in fact lost The Mariners were all relatively small robotic during launch, but their backups were successful. No explorers, each launched on an Atlas rocket with Mariners were lost in later flight to their destination either an Agena or Centaur upper-stage booster, and planets or before completing their scientific missions. -
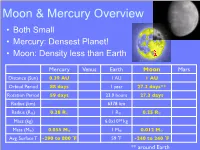
Moon & Mercury Overview
Moon & Mercury Overview • Both Small • Mercury: Densest Planet! • Moon: Density less than Earth Mercury Venus Earth Moon Mars Distance (Sun) 0.39 AU 1 AU 1 AU Orbital Period 88 days 1 year 27.3 days** Rotation Period 59 days 23.9 hours 27.3 days Radius (km) 6378 km Radius (R⊕) 0.38 R⊕ 1 R⊕ 0.25 R⊕ Mass (kg) 6.0x1024 kg Mass (M⊕) 0.055 M⊕ 1 M⊕ 0.012 M⊕ Avg. Surface T -290 to 800 F̊ 59 F̊ -240 to 240 F̊ ** around Earth Lunar Surface • Cratered – 30,000 big (> 1km) – millions of smaller craters MercurianMercury Surface - Surface Features Messenger Images Era of Heavy Bombardment Period of intense impacts in the inner S.S. by leftover planetesimals from outer S.S. 4.1-3.8 billion yrs ago affects all Terr. planets Lunar Surface • Dark Maria (“Seas”) • Lighter Highlands (“Lands”, Terrae) Lunar Surface • Maria What’s different? Why? • Highlands Apollo Lunar Surface Maria: smooth, flat, fewer craters Highlands: VERY cratered Maria have been resurfaced! Maria Formation After Heavy Bombardment Volcanic Activity caused by heat from radioactive decay flooded old craters Dating the Lunar Surface Crater Counting! Which is oldest? The Moon - Space Exploration • Luna 3 (Russia), 1959 Luna 3 farside • NASA Rangers (Impactors), 1961-1965 • Orbiters (99% mapped), 1966-1967 • Surveyors (landers), 1966-1968 • Apollo (manned), 1968-1972 • Clementine (Multi-wavel. mapping), 1994 • Prospector (polar orbiter), 1998 • SMART-1 (ESA Impactor), 2003-2006 • Kaguya (Japan), 2007- Prospector • Chang-e 1 (China), 2007- • Chandrayaan (India), 2008- Orbiter • Lunar Recon. -

The Composition of Planetary Atmospheres 1
The Composition of Planetary Atmospheres 1 All of the planets in our solar system, and some of its smaller bodies too, have an outer layer of gas we call the atmosphere. The atmosphere usually sits atop a denser, rocky crust or planetary core. Atmospheres can extend thousands of kilometers into space. The table below gives the name of the kind of gas found in each object’s atmosphere, and the total mass of the atmosphere in kilograms. The table also gives the percentage of the atmosphere composed of the gas. Object Mass Carbon Nitrogen Oxygen Argon Methane Sodium Hydrogen Helium Other (kilograms) Dioxide Sun 3.0x1030 71% 26% 3% Mercury 1000 42% 22% 22% 6% 8% Venus 4.8x1020 96% 4% Earth 1.4x1021 78% 21% 1% <1% Moon 100,000 70% 1% 29% Mars 2.5x1016 95% 2.7% 1.6% 0.7% Jupiter 1.9x1027 89.8% 10.2% Saturn 5.4x1026 96.3% 3.2% 0.5% Titan 9.1x1018 97% 2% 1% Uranus 8.6x1025 2.3% 82.5% 15.2% Neptune 1.0x1026 1.0% 80% 19% Pluto 1.3x1014 8% 90% 2% Problem 1 – Draw a pie graph (circle graph) that shows the atmosphere constituents for Mars and Earth. Problem 2 – Draw a pie graph that shows the percentage of Nitrogen for Venus, Earth, Mars, Titan and Pluto. Problem 3 – Which planet has the atmosphere with the greatest percentage of Oxygen? Problem 4 – Which planet has the atmosphere with the greatest number of kilograms of oxygen? Problem 5 – Compare and contrast the objects with the greatest percentage of hydrogen, and the least percentage of hydrogen. -

Mercury Spill Fact Sheet
This fact sheet is intended to be a quick reference tool for people who are involved in a mercury spill and cleanup event. MERCURY Mercury mercury MERCURY MERCURY MERCURY Elemental mercury, also called “quicksilver,” is a heavy, silvery, form of the metal mercury that is liquid at room temperature. It can slowly change from a liquid into a gas that is invisible to the naked eye. The gas or “vapors” that are released will start to fill a room if mercury is spilled indoors. MERCURY EXPOSURE Mercury is a very toxic or poisonous substance that people can be exposed to in several ways. If it is swallowed, like from a broken thermometer, it mostly passes through your body and very little is absorbed. If you touch it, a small amount may pass through your skin, but not usually enough to harm you. Mercury is most harmful when you breathe in the vapors that are released when a container is open or a spill occurs. HEALTH EFFECTS Pregnant women, infants and young children are particularly sensitive to the harmful effects of mercury. The health effects that can result from mercury exposure depend on how much mercury you are exposed to and how long you are exposed. Some of the acute effects, ones that may come soon after exposure to high concentrations of mercury are: • Headaches, chills, fever • chest tightness, coughs • hand tremors • nausea, vomiting, abdominal cramps, diarrhea Some effects that may result from chronic, or longer term exposure to mercury vapor can be: • Personality changes • Decreased vision or hearing • Peripheral nerve damage • Elevated blood pressure Children are especially sensitive to mercury and at risk of developing a condition known as acrodynia or “Pinks Disease” by breathing vapors or other exposure circumstances. -

Elemental Mercury Emission in the Indoor Environment Due to Broken Compact Fluorescent Light (CFL) Bulbs
Elemental Mercury Emission In The Indoor Environment Due To Broken Compact Fluorescent Light (CFL) Bulbs David Marr1, Mark Mason1, Stanley Durkee2 1U.S. EPA, RTP, NC 2U.S. EPA, Washington DC Mark Mason l [email protected] l 919-541-4835 Paper control number 553 Introduction Mercury emissions from broken Broken CFL Bulb Cleanup fluorescent bulbs Compact fluorescent light (CFL) bulbs require elemental The U.S EPA, as part of its public outreach program, provides mercury for operation. Each bulb contains a few milligrams Published research on mercury emissions cleanup guidance when a CFL is broken in the indoor of mercury, a value that has been decreasing over time due to environment. As part of this guidance, it is recommended that new technologies and methodologies, driven primarily by from lighting the debris be cleaned up following 5 to 10 minutes of increased environmental and consumer concerns. If a CFL breaks, Aucott et al. (2003) generated mercury emission rate ventilation and some initial steps. Figure 3 shows indoor model some of the mercury is immediately released as elemental equations for fluorescent lamps (FLs) broken in a barrel at results that include the emission rate of Equation 1, but with mercury vapor while the remainder is available for emission three ambient air temperatures in an effort to quantify source removal occurring at 15 minutes to provide a visual of over time to air via the bulb debris and contaminated indoor mercury emissions in time. Equation 1 is their published the impact of proper cleanup on indoor mercury concentrations surfaces until properly remediated. -

Mercury Use and Loss from Gold Mining in Nineteenth-Century Victoria 45
CSIRO Publishing The Royal Society of Victoria, 127, 44 –54, 2015 www.publish.csiro.au/journals/rs 10.1071/RS15017 Me RCuRy uSe and LOSS fROM GOLd MInInG In nIneTeenTh- CenTuRy Victoria Peter Davies1, susan Lawrence2 anD JoDi turnbuLL3 1, 2, 3 department of archaeology and history, La Trobe university, Bundoora, Victoria 3086 Correspondence: Peter davies, [email protected] ABSTRACT: This paper reports on preliminary research into gold-mining-related mercury contamination in nineteenth-century Victoria. data drawn from contemporary sources, including Mineral Statistics of Victoria and Mining Surveyors Reports from 1868‒1888, are used to calculate quantities of mercury used by miners to amalgamate gold in stamp batteries and the rates of mercury lost in the process. Some of the mercury discharged from mining and ore milling flowed into nearby waterways and some remained in the waste residue, the tailings near the mills. We estimate that a minimum of 121 tons of mercury were discharged from stamp batteries in this period. although the figures fluctuate through time and space, they allow a good estimate of how much mercury was leaving the mine workings and entering Victorian creeks and rivers. Better understanding of historic mercury loss can provide the basis for improved mapping of mercury distribution in modern waterways, which can in turn inform the management of catchment systems. Keywords: mercury, gold mining, pollution, water, rivers In recent years, mercury in waterways has emerged as an et al. 1996) and new Zealand (Moreno et al. 2005), while important environmental issue in south-eastern australia extensive mercury pollution also resulted from silver and in many other areas.