Analysis of Pesticide Residues in Mango by GC/MS/MS with Bond Elut Quechers EN Kits
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2,4-Dichlorophenoxyacetic Acid
2,4-Dichlorophenoxyacetic acid 2,4-Dichlorophenoxyacetic acid IUPAC (2,4-dichlorophenoxy)acetic acid name 2,4-D Other hedonal names trinoxol Identifiers CAS [94-75-7] number SMILES OC(COC1=CC=C(Cl)C=C1Cl)=O ChemSpider 1441 ID Properties Molecular C H Cl O formula 8 6 2 3 Molar mass 221.04 g mol−1 Appearance white to yellow powder Melting point 140.5 °C (413.5 K) Boiling 160 °C (0.4 mm Hg) point Solubility in 900 mg/L (25 °C) water Related compounds Related 2,4,5-T, Dichlorprop compounds Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.[1] 2,4-D is also an important synthetic auxin, often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. History 2,4-D was developed during World War II by a British team at Rothamsted Experimental Station, under the leadership of Judah Hirsch Quastel, aiming to increase crop yields for a nation at war.[citation needed] When it was commercially released in 1946, it became the first successful selective herbicide and allowed for greatly enhanced weed control in wheat, maize (corn), rice, and similar cereal grass crop, because it only kills dicots, leaving behind monocots. Mechanism of herbicide action 2,4-D is a synthetic auxin, which is a class of plant growth regulators. -

Routine Multipesticide Analysis Orbitrap
Routine Multi-Pesticide Residue Analysis by Orbitrap MS Technology Osama Abu-Nimreh CMD Sales Support Specialist MECEC , Dubai The world leader in serving science Challenges of Pesticide-Residues Analysis • Sample variability (matrix) • Different compound characteristics • Large number of samples • Hundreds of analytes monitored • Low levels controlled • Baby food (MRL for all pesticides = 0.01 mg/kg) • Fast response required 2 Former Pesticide Multi-Residue Method Setup . Extraction Acetonitrile, Ethyl Mostly replaced acetate, Methanol... by QuEChERS today . Clean-up GPC, SPE, LLE, LC . Determination GC, LC, GC-MS, LC-MS, Thermo Scientific™ QuEChERS™ GC-MS/MS, LC-MS/MS... method 3 Simplified Extraction Procedure Applied 10 g of sample is weighed into Quechers extraction tube + 20 mL of water + 10 mL of ACN shaking 10 min Centrifugation 5 min @ 5000 rpm Injection to LC-HRAM 4 Consumables Used Consumables/Chemicals Part Number Acetonitrile A/0638/17 QuEChERS extraction tube, 50 mL, 250 pack 60105-216 QuEChERS pouches, 50 pack 60105-344 Apparatus/Columns Part Number Horizontal shaker 1069-3391 Horizontal shaker plate 1053-0102 Thermo Scientific™ Barnstead™ EASYpure™II water 3125753 Thermo Scientific™ Heraeus™ Fresco™ 17 micro centrifuge 3208590 Thermo Scientific™ Accucore™ aQ column 100x2.1, 2.6 µm 17326-102130 5 Improving QuEChERS Extraction Tips & Tricks: • Dry food (cereals/dried food, < 25 % water content): • Addition of water to enable adequate partitioning and reducing interaction of pesticides with matrix • Food containing fat/wax (avocado/oil): • After extraction step add a freezing out step and transfer supernatant to clean-up tube • More clean-up might be needed of raw extract (PSA+C18) • Food containing complex matrix (tea/spices) • Additional clean-up with GCB might be necessary (potential loss of planar structure pesticides like thiabendazole) • Acidic food (citrus): • Adjust pH (5-5.5) to increase recovery (e.g. -

Novel Approach to Fast Determination of 64 Pesticides Using of Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry
Novel approach to fast determination of 64 pesticides using of ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) Tomas Kovalczuk, Ondrej Lacina, Martin Jech, Jan Poustka, Jana Hajslova To cite this version: Tomas Kovalczuk, Ondrej Lacina, Martin Jech, Jan Poustka, Jana Hajslova. Novel approach to fast determination of 64 pesticides using of ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Food Additives and Contaminants, 2008, 25 (04), pp.444-457. 10.1080/02652030701570156. hal-00577414 HAL Id: hal-00577414 https://hal.archives-ouvertes.fr/hal-00577414 Submitted on 17 Mar 2011 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Food Additives and Contaminants For Peer Review Only Novel approach to fast determination of 64 pesticides using of ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) Journal: Food Additives and Contaminants Manuscript ID: TFAC-2007-065.R1 Manuscript Type: Original Research Paper Date Submitted by the 07-Jul-2007 Author: Complete List of Authors: -

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Herbicide Mode of Action Table High Resistance Risk
Herbicide Mode of Action Table High resistance risk Chemical family Active constituent (first registered trade name) GROUP 1 Inhibition of acetyl co-enzyme A carboxylase (ACC’ase inhibitors) clodinafop (Topik®), cyhalofop (Agixa®*, Barnstorm®), diclofop (Cheetah® Gold* Decision®*, Hoegrass®), Aryloxyphenoxy- fenoxaprop (Cheetah®, Gold*, Wildcat®), fluazifop propionates (FOPs) (Fusilade®), haloxyfop (Verdict®), propaquizafop (Shogun®), quizalofop (Targa®) Cyclohexanediones (DIMs) butroxydim (Factor®*), clethodim (Select®), profoxydim (Aura®), sethoxydim (Cheetah® Gold*, Decision®*), tralkoxydim (Achieve®) Phenylpyrazoles (DENs) pinoxaden (Axial®) GROUP 2 Inhibition of acetolactate synthase (ALS inhibitors), acetohydroxyacid synthase (AHAS) Imidazolinones (IMIs) imazamox (Intervix®*, Raptor®), imazapic (Bobcat I-Maxx®*, Flame®, Midas®*, OnDuty®*), imazapyr (Arsenal Xpress®*, Intervix®*, Lightning®*, Midas®* OnDuty®*), imazethapyr (Lightning®*, Spinnaker®) Pyrimidinyl–thio- bispyribac (Nominee®), pyrithiobac (Staple®) benzoates Sulfonylureas (SUs) azimsulfuron (Gulliver®), bensulfuron (Londax®), chlorsulfuron (Glean®), ethoxysulfuron (Hero®), foramsulfuron (Tribute®), halosulfuron (Sempra®), iodosulfuron (Hussar®), mesosulfuron (Atlantis®), metsulfuron (Ally®, Harmony®* M, Stinger®*, Trounce®*, Ultimate Brushweed®* Herbicide), prosulfuron (Casper®*), rimsulfuron (Titus®), sulfometuron (Oust®, Eucmix Pre Plant®*, Trimac Plus®*), sulfosulfuron (Monza®), thifensulfuron (Harmony®* M), triasulfuron (Logran®, Logran® B-Power®*), tribenuron (Express®), -

40 CFR Ch. I (7–1–18 Edition) § 455.61
§ 455.61 40 CFR Ch. I (7–1–18 Edition) from: the operation of employee show- § 455.64 Effluent limitations guidelines ers and laundry facilities; the testing representing the degree of effluent of fire protection equipment; the test- reduction attainable by the applica- ing and emergency operation of safety tion of the best available tech- showers and eye washes; or storm nology economically achievable water. (BAT). (d) The provisions of this subpart do Except as provided in 40 CFR 125.30 not apply to wastewater discharges through 125.32, any existing point from the repackaging of microorga- source subject to this subpart must nisms or Group 1 Mixtures, as defined achieve effluent limitations rep- under § 455.10, or non-agricultural pes- resenting the degree of effluent reduc- ticide products. tion attainable by the application of the best available technology economi- § 455.61 Special definitions. cally achievable: There shall be no dis- Process wastewater, for this subpart, charge of process wastewater pollut- means all wastewater except for sani- ants. tary water and those wastewaters ex- § 455.65 New source performance cluded from the applicability of the standards (NSPS). rule in § 455.60. Any new source subject to this sub- § 455.62 Effluent limitations guidelines part which discharges process waste- representing the degree of effluent water pollutants must meet the fol- reduction attainable by the applica- lowing standards: There shall be no dis- tion of the best practicable pollut- charge of process wastewater pollut- ant control technology (BPT). ants. Except as provided in 40 CFR 125.30 through 125.32, any existing point § 455.66 Pretreatment standards for existing sources (PSES). -

Development of a CEN Standardised Method for Liquid Chromatography Coupled to Accurate Mass Spectrometry
Development of a CEN standardised method for liquid chromatography coupled to accurate mass spectrometry CONTENTS 1. Aim and scope ................................................................................................................. 2 2. Short description ................................................................................................................ 2 3. Apparatus and consumables ......................................................................................... 2 4. Chemicals ........................................................................................................................... 2 5. Procedure ........................................................................................................................... 3 5.1. Sample preparation ................................................................................................... 3 5.2. Recovery experiments for method validation ...................................................... 3 5.3. Extraction method ...................................................................................................... 3 5.4. Measurement .............................................................................................................. 3 5.5. Instrumentation and analytical conditions ............................................................ 4 5.5.1. Dionex Ultimate 3000 .......................................................................................... 4 5.5.2. QExactive Focus HESI source parameters ..................................................... -

INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES
US Environmental Protection Agency Office of Pesticide Programs INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES Note: Pesticide tolerance information is updated in the Code of Federal Regulations on a weekly basis. EPA plans to update these indexes biannually. These indexes are current as of the date indicated in the pdf file. For the latest information on pesticide tolerances, please check the electronic Code of Federal Regulations (eCFR) at http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfrv23_07.html 1 40 CFR Type Family Common name CAS Number PC code 180.163 Acaricide bridged diphenyl Dicofol (1,1-Bis(chlorophenyl)-2,2,2-trichloroethanol) 115-32-2 10501 180.198 Acaricide phosphonate Trichlorfon 52-68-6 57901 180.259 Acaricide sulfite ester Propargite 2312-35-8 97601 180.446 Acaricide tetrazine Clofentezine 74115-24-5 125501 180.448 Acaricide thiazolidine Hexythiazox 78587-05-0 128849 180.517 Acaricide phenylpyrazole Fipronil 120068-37-3 129121 180.566 Acaricide pyrazole Fenpyroximate 134098-61-6 129131 180.572 Acaricide carbazate Bifenazate 149877-41-8 586 180.593 Acaricide unclassified Etoxazole 153233-91-1 107091 180.599 Acaricide unclassified Acequinocyl 57960-19-7 6329 180.341 Acaricide, fungicide dinitrophenol Dinocap (2, 4-Dinitro-6-octylphenyl crotonate and 2,6-dinitro-4- 39300-45-3 36001 octylphenyl crotonate} 180.111 Acaricide, insecticide organophosphorus Malathion 121-75-5 57701 180.182 Acaricide, insecticide cyclodiene Endosulfan 115-29-7 79401 -

4. Chemical and Physical Information
PYRETHRINS AND PYRETHROIDS 131 4. CHEMICAL AND PHYSICAL INFORMATION 4.1 CHEMICAL IDENTITY The naturally-occurring pyrethrins, extracted from chrysanthemum flowers, are esters of chrysanthemic acid (Pyrethrin I, Cinerin I, and Jasmolin I) and esters of pyrethric acid (Pyrethrin II, Cinerin II, and Jasmolin II). In the United States, the pyrethrum extract is standardized as 45–55% w/w total pyrethrins. The typical proportion of Pyrethrins I to II is 0.2:2.8, while the ratio of pyrethrins:cinerins:jasmolins is 71:21:7 (Tomlin 1997). Information regarding the chemical identity of the pyrethrins is presented in Table 4-1. Pyrethroids are synthetic esters derived from the naturally-occurring pyrethrins. One exception to the axiom that all pyrethroids are esters of carboxylic acids is noteworthy. There is a group of oxime ethers that exhibits insecticidal activity similar in nature to the pyrethrins and pyrethroid esters (Davies 1985). Little data exist regarding these compounds, and no commercial products have been produced. Commercially available pyrethroids include allethrin, bifenthrin, bioresmethrin, cyfluthrin, cyhalothrin, cypermethrin, deltamethrin, esfenvalerate (fenvalerate), flucythrinate, flumethrin, fluvalinate, fenpropathrin, permethrin, phenothrin, resmethrin, tefluthrin, tetramethrin, and tralomethrin. Information regarding the chemical identity of pyrethroids is shown in Table 4-2. With the exception of deltamethrin, pyrethroids are a complex mixture of isomers rather than one single pure compound. For pyrethroids possessing the cyclopropane moiety, isomerism about the cyclopropane ring greatly influences the toxicity of these insecticides. The presence of two chiral centers in the ring results in two pairs of diastereomers. The diastereomers and their nonsuperimposable mirror images (enantiomers) are illustrated in Figure 4-1. -

Validation Report 28
EURL for Cereals and Feeding stuff National Food Institute Technical University of Denmark Validation Report 28 Determination of pesticide residues in hay by LC-MS/MS and GC-MS/MS (QuEChERS method) Susan Strange Herrmann Mette Erecius Poulsen February 2018 Page 2 of 67 CONTENT: 1. Introduction ...................................................................................................................................... 3 2. Principle of analysis......................................................................................................................... 3 3. Validation design ............................................................................................................................. 4 4. Calibration curves............................................................................................................................ 4 5. Validation parameters...................................................................................................................... 4 6. Criteria for the acceptance of validation results ............................................................................. 5 7. Results and conclusion ..................................................................................................................... 6 9. References ........................................................................................................................................ 6 Appendix 1a. GCMSMS transitions used for validation of pesticides in Hay .................................... -

Quantitative Determination of Pyrethroids, Pyrethrins, and Piperonyl Butoxide in Surface Water by High-Resolution Gas Chromatography/High-Resolution Mass Spectrometry
J. Agric. Food Chem. 2006, 54, 6957−6962 6957 Quantitative Determination of Pyrethroids, Pyrethrins, and Piperonyl Butoxide in Surface Water by High-Resolution Gas Chromatography/High-Resolution Mass Spectrometry MILLION BEKELE WOUDNEH* AXYS Analytical Services Ltd., 2045 Mills Road West, Sidney, British Columbia V8L 3S8, Canada DANIEL RAY OROS San Francisco Estuary Institute, 7770 Pardee Lane, 2nd Floor, Oakland, California 94621 A new method for determination of pyrethroids, pyrethrins, and piperonyl butoxide (PBO) by high- resolution gas chromatography/high-resolution mass spectrometry (HRGC/HRMS) was developed for surface water samples. The method is based on sampling 100 L of ambient surface water with a solid phase extraction (SPE) technique that uses both wound glass fiber filters for collecting the particulate-associated chemicals and XAD-2 resin for collecting the dissolved chemicals. The method detection limits of the analytes ranged from 0.58 to 8.16 ng/sample, which is equivalent to a detection limit range of 0.0058-0.082 ng/L for a 100 L water sample collected by the SPE technique. The SPE when coupled with HRGC/HRMS was a suitable match for detecting these chemicals at subnanogram per liter ranges that are toxicologically significant to aquatic organisms. To confirm the utility of this method for environmental applications, pyrethroids and PBO were found at subnanogram per liter concentrations in surface water samples collected from five tributaries (primarily urban creeks) of the San Francisco Bay, California. KEYWORDS: Pyrethroids; pesticides; XAD-2; HRGC/HRMS; San Francisco Bay INTRODUCTION by inhibiting a group of enzymes (mixed-function oxidases) that are involved in pyrethroid detoxification, which as a result The decision of the U.S. -
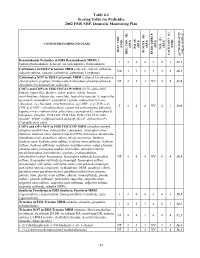
2002 NRP Section 6, Tables 6.1 Through
Table 6.1 Scoring Table for Pesticides 2002 FSIS NRP, Domestic Monitoring Plan } +1 0.05] COMPOUND/COMPOUND CLASS * ) (EPA) (EPA) (EPA) (EPA) (EPA) (FSIS) (FSIS) PSI (P) TOX.(T) L-1 HIST. VIOL. BIOCON. (B) {[( (2*R+P+B)/4]*T} REG. CON. (R) * ENDO. DISRUP. LACK INFO. (L) LACK INFO. {[ Benzimidazole Pesticides in FSIS Benzimidazole MRM (5- 131434312.1 hydroxythiabendazole, benomyl (as carbendazim), thiabendazole) Carbamates in FSIS Carbamate MRM (aldicarb, aldicarb sulfoxide, NA44234416.1 aldicarb sulfone, carbaryl, carbofuran, carbofuran 3-hydroxy) Carbamates NOT in FSIS Carbamate MRM (carbaryl 5,6-dihydroxy, chlorpropham, propham, thiobencarb, 4-chlorobenzylmethylsulfone,4- NT 4 1 3 NV 4 4 13.8 chlorobenzylmethylsulfone sulfoxide) CHC's and COP's in FSIS CHC/COP MRM (HCB, alpha-BHC, lindane, heptachlor, dieldrin, aldrin, endrin, ronnel, linuron, oxychlordane, chlorpyrifos, nonachlor, heptachlor epoxide A, heptachlor epoxide B, endosulfan I, endosulfan I sulfate, endosulfan II, trans- chlordane, cis-chlordane, chlorfenvinphos, p,p'-DDE, p, p'-TDE, o,p'- 3444NV4116.0 DDT, p,p'-DDT, carbophenothion, captan, tetrachlorvinphos [stirofos], kepone, mirex, methoxychlor, phosalone, coumaphos-O, coumaphos-S, toxaphene, famphur, PCB 1242, PCB 1248, PCB 1254, PCB 1260, dicofol*, PBBs*, polybrominated diphenyl ethers*, deltamethrin*) (*identification only) COP's and OP's NOT in FSIS CHC/COP MRM (azinphos-methyl, azinphos-methyl oxon, chlorpyrifos, coumaphos, coumaphos oxon, diazinon, diazinon oxon, diazinon met G-27550, dichlorvos, dimethoate, dimethoate