Magnitude and Extent of Chemical Contamination and Toxicity in Sediments of Biscayne Bay and Vicinity. US Department of Commerce
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Initial Draft – for Discussion Purposes Only
Initial Draft – For Discussion Purposes Only Draft South Florida Canal Aquatic Life Study October 29, 2012 1 Initial Draft – For Discussion Purposes Only Draft South Florida Canal Aquatic Life Study Background and Introduction The Central & Southern Florida (C&SF) Project, which was authorized by Congress in 1948, has dramatically altered the waters of south Florida. The current C&SF Project includes 2600 miles of canals, over 1300 water control structures, and 64 pump stations1. The C&SF Project, which is operated by the South Florida Water Management District (SFWMD), provides water supply, flood control, navigation, water management, and recreational benefits to south Florida. As a part of the C&SF, there are four major canals running from Lake Okeechobee to the lower east coast – the West Palm Beach Canal (42 miles long), Hillsboro Canal (51 miles), North New River Canal (58 miles) and Miami canal (85 miles). In addition, there are many more miles of primary, secondary and tertiary canals operated as a part of or in conjunction with the C&SF or as a part of other water management facilities within the SFWMD. Other entities operating associated canals include counties and special drainage districts. There is a great deal of diversity in the design, construction and operation of these canals. The hydrology of the canals is highly manipulated by a series of water control structures and levees that have altered the natural hydroperiods and flows of the South Florida watershed on regional to local scales. Freshwater and estuarine reaches of water bodies are delineated by coastal salinity structures operated by the SFWMD. -
Of Surface-Water Records to September 30, 1955
GEOLOGICAL SURVEY CIRCULAR 382 INDEX OF SURFACE-WATER RECORDS TO SEPTEMBER 30, 1955 PART 2. SOUTH ATLANTIC SLOPE AND EASTERN GULF OF MEXICO BASINS UNITED STATES DEPARTMENT OF THE INTERIOR Fred A. Seaton, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director GEOLOGICAL SURVEY CIRCULAR 382 INDEX OF SURFACE-WATER RECORDS TO SEPTEMBER 30,1955 PART 2. SOUTH ATLANTIC SLOPE AND EASTERN GULF OF MEXICO BASINS By P. R. Speer and A. B. Goodwin Washington, D. C., 1956 Free on application to the Geological Survey, Washington 25, D. C. INDEX OF SURFACE-WATER RECORDS TO SEPTEMBER 30,1955 PAET 2. SOUTH ATLANTIC SLOPE AND EASTERN GULF OF MEXICO BASINS By P. R Speer and A. B. Goodwin EXPLANATION This index lists the streamflow and reservoir stations in the South Atlantic slope and Eastern Gulf of Mexico basins for which records have been or are to be published in reports of the Geological Survey for periods prior to September 30, 1955. Periods of record for the same station published by other agencies are listed only when they contain more detailed information or are for periods not reported in publications of the Geological Survey. The stations are listed in the downstream order first adopted for use in the 1951 series of water-supply papers on surface-water supply of the United States. Starting at the headwater of each stream all stations are listed in a downstream direction. Tributary streams are indicated by indention and are inserted between main-stem stations in the order in which they enter the main stream. To indicate the rank of any tributary on which a record is available and the stream to which it is immediately tributary, each indention in the listing of stations represents one rank. -

Wilderness on the Edge: a History of Everglades National Park
Wilderness on the Edge: A History of Everglades National Park Robert W Blythe Chicago, Illinois 2017 Prepared under the National Park Service/Organization of American Historians cooperative agreement Table of Contents List of Figures iii Preface xi Acknowledgements xiii Abbreviations and Acronyms Used in Footnotes xv Chapter 1: The Everglades to the 1920s 1 Chapter 2: Early Conservation Efforts in the Everglades 40 Chapter 3: The Movement for a National Park in the Everglades 62 Chapter 4: The Long and Winding Road to Park Establishment 92 Chapter 5: First a Wildlife Refuge, Then a National Park 131 Chapter 6: Land Acquisition 150 Chapter 7: Developing the Park 176 Chapter 8: The Water Needs of a Wetland Park: From Establishment (1947) to Congress’s Water Guarantee (1970) 213 Chapter 9: Water Issues, 1970 to 1992: The Rise of Environmentalism and the Path to the Restudy of the C&SF Project 237 Chapter 10: Wilderness Values and Wilderness Designations 270 Chapter 11: Park Science 288 Chapter 12: Wildlife, Native Plants, and Endangered Species 309 Chapter 13: Marine Fisheries, Fisheries Management, and Florida Bay 353 Chapter 14: Control of Invasive Species and Native Pests 373 Chapter 15: Wildland Fire 398 Chapter 16: Hurricanes and Storms 416 Chapter 17: Archeological and Historic Resources 430 Chapter 18: Museum Collection and Library 449 Chapter 19: Relationships with Cultural Communities 466 Chapter 20: Interpretive and Educational Programs 492 Chapter 21: Resource and Visitor Protection 526 Chapter 22: Relationships with the Military -

Current Status of Oyster Reefs in Florida Waters: Knowledge and Gaps
Current Status of Oyster Reefs in Florida Waters: Knowledge and Gaps Dr. William S. Arnold Florida FWC Fish and Wildlife Research Lab 100 Eighth Avenue SE St. Petersburg, FL 33701 727-896-8626 [email protected] Outline • History-statewide distribution • Present distribution – Mapped populations and gaps – Methodological variation • Ecological status • Application Need to Know Ecological value of oyster reefs will be clearly defined in subsequent talks Within “my backyard”, at least some idea of need to protect and preserve, as exemplified by the many reef restoration projects However, statewide understanding of status and trends is poorly developed Culturally important- archaeological evidence suggests centuries of usage Long History of Commercial Exploitation US Landings (Lbs of Meats x 1000) 80000 70000 60000 50000 40000 30000 20000 10000 0 1950 1960 1970 1980 1990 2000 Statewide: Economically important: over $2.8 million in landings value for Florida fishery in 2003 Most of that value is from Franklin County (Apalachicola Bay), where 3000 landings have been 2500 2000 relatively stable since 1985 1500 1000 In other areas of state, 500 0 oysters landings are on 3000 decline due to loss of 2500 Franklin County 2000 access, degraded water 1500 quality, and loss of oyster 1000 populations 500 0 3000 Panhandle other 2500 2000 1500 1000 Pounds500 of Meats (x 1000) 0 3000 Peninsular West Coast 2500 2000 1500 1000 500 0 Peninsular East Coast 1985 1986 1987 1988 1989 1990 1991 1992 1993 Year 1994 1995 1996 1997 1998 1999 2000 MAPPING Tampa Bay Oyster Maps More reef coverage than anticipated, but many of the reefs are moderately to severely degraded Kathleen O’Keife will discuss Tampa Bay oyster mapping methods in the next talk Caloosahatchee River and Estero Bay Aerial imagery used to map reefs, verified by ground-truthing Southeast Florida oyster maps • Used RTK-GPS equipment to map in both the horizontal and the vertical. -
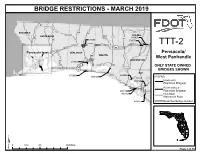
Ttt-2-Map.Pdf
BRIDGE RESTRICTIONS - MARCH 2019 <Double-click here to enter title> «¬89 4 2 ESCAMBIA «¬ «¬189 85 «¬ «¬ HOLMES 97 SANTA ROSA ¬« 29 331187 83 610001 ¤£ ¤£«¬ «¬ 81 87 570006 «¬ «¬ 520076 TTT-2 10 ¦¨§ ¤£90 «¬79 Pensacola Inset OKALOOSA Pensacola/ «¬285 WALTON «¬77 West Panhandle 293 WASHINGTON «¬87 570055 ¦¨§ ONLY STATE OWNED 20 ¤£98 «¬ BRIDGES SHOWN BAY 570082 460051 600108 LEGEND 460020 Route with «¬30 Restricted Bridge(s) 368 Route without 460113 «¬ Restricted Bridge(s) 460112 Non-State Maintained Road 460019 ######Restricted Bridge Number 0 12.5 25 50 Miles ¥ Page 1 of 16 BRIDGE RESTRICTIONS - MARCH 2019 <Double-click here to enter title> «¬2 HOLMES JACKSON 610001 71 530005 520076 «¬ «¬69 TTT-2 ¬79 « ¤£90 Panama City/ «¬77 ¦¨§10 GADSDEN ¤£27 WASHINGTON JEFFERSON Tallahassee 500092 ¤£19 ONLY STATE OWNED ¬20 BRIDGES SHOWN BAY « CALHOUN 460051 «¬71 «¬65 Tallahassee Inset «¬267 231 73 LEGEND ¤£ «¬ LEON 59 «¬ Route with Restricted Bridge(s) 460020 LIBERTY 368 «¬ Route without WAKULLA 61 «¬22 «¬ Restricted Bridge(s) 98 460112 ¤£ Non-State 460113 Maintained Road 460019 GULF TA ###### Restricted Bridge Number 98 FRANKLIN ¤£ 490018 ¤£319 «¬300 490031 0 12.5 25 50 Miles ¥ Page 2 of 16 BRIDGE RESTRICTIONS - MARCH 2019 350030 <Double-click320017 here to enter title> JEFFERSON «¬53 «¬145 ¤£90 «¬2 «¬6 HAMILTON COLUMBIA ¦¨§10 290030 «¬59 ¤£441 19 MADISON BAKER ¤£ 370013 TTT-2 221 ¤£ SUWANNEE ¤£98 ¤£27 «¬247 Lake City TAYLOR UNION 129 121 47 «¬ ¤£ ¬ 238 ONLY STATE OWNED « «¬ 231 LAFAYETTE «¬ ¤£27A BRIDGES SHOWN «¬100 BRADFORD LEGEND 235 «¬ Route with -

State of Emergency on Red Tide for Tampa Bay
July 19, 2021 Governor Ron DeSantis State of Florida The Capitol 400 S. Monroe St. Tallahassee, FL 32399-0001 [email protected] Re: State of Emergency on Red Tide for Tampa Bay The undersigned respectfully request you immediately declare a state of emergency for the ongoing red tide and fish kill occurring in Tampa Bay. Such a declaration would help coordinate and fund relief efforts to mitigate further environmental and economic damage from red tide in the region. Red tide produces toxic chemicals that harm marine wildlife and humans. The ongoing, widespread red tide and fish kills have unreasonably interfered with the health, safety, and welfare of the State of Florida, causing harm to its environment and fragile ecosystems in Hillsborough, Manatee, Pinellas, and Sarasota counties. Therefore, we ask you exercise your authority, as the Governor of Florida, vested by the Florida Constitution and the Florida Emergency Management Act to issue an executive order declaring a state of emergency due to red tide in Tampa Bay. Sincerely, Audubon Everglades Scott Zucker, President [email protected] Cape Coral Friends of Wildlife Paul Bonasia, President [email protected] Cape Coral Wildlife Trust Lori Haus-Bulcock [email protected] Calusa Waterkeeper John Cassani, Calusa Waterkeeper [email protected] Cat Chase Media Caitlin Chase, Owner [email protected] Center for Biological Diversity Elise Bennett, Staff Attorney [email protected] Chispa Florida Maria Revelles, Program Director [email protected] Collins Law Group Martha M. Collins, Esq. [email protected] Defenders of Wildlife Elizabeth Flemming, Senior Florida Representative [email protected] Environment Florida Jenna Stevens, State Director [email protected] Florida Student Power Network Mary-Elizabeth Estrada, Tampa Climate Justice Organizer [email protected] Florida Turtle Conservation Trust George L. -

Collier Miami-Dade Palm Beach Hendry Broward Glades St
Florida Fish and Wildlife Conservation Commission F L O R ID A 'S T U R N P IK E er iv R ee m Lakewood Park m !( si is O K L D INDRIO ROAD INDRIO RD D H I N COUNTY BCHS Y X I L A I E O W L H H O W G Y R I D H UCIE BLVD ST L / S FT PRCE ILT SRA N [h G Fort Pierce Inlet E 4 F N [h I 8 F AVE "Q" [h [h A K A V R PELICAN YACHT CLUB D E . FORT PIERCE CITY MARINA [h NGE AVE . OKEECHOBEE RA D O KISSIMMEE RIVER PUA NE 224 ST / CR 68 D R !( A D Fort Pierce E RD. OS O H PIC R V R T I L A N N A M T E W S H N T A E 3 O 9 K C A R-6 A 8 O / 1 N K 0 N C 6 W C W R 6 - HICKORY HAMMOCK WMA - K O R S 1 R L S 6 R N A E 0 E Lake T B P U Y H D A K D R is R /NW 160TH E si 68 ST. O m R H C A me MIDWAY RD. e D Ri Jernigans Pond Palm Lake FMA ver HUTCHINSON ISL . O VE S A t C . T I IA EASY S N E N L I u D A N.E. 120 ST G c I N R i A I e D South N U R V R S R iv I 9 I V 8 FLOR e V ESTA DR r E ST. -

Market Assessment Sources
To: Elizabeth Abernethy, Director, Date: October 2020 Planning and Development Services City of St. Petersburg Project #: 66316.00 From: Neale Stralow, Senior Planner Re: StPete2050 - Market Assessment Sources This correspondence is provided at the request of City of St. Petersburg staff relating to the final Market Assessment reporting provided by Landwise Advisors, LLC dated January 24, 2020 in support of the StPete2050 project. The final submittal includes source references and responses to City staff provided comments. The following is a source listing for future use by City staff. Slide # - Description Stated Sources Slide 5 – SWOT Grow Smarter 2014 and update 2019 State of the City, 2019 StPete2050 Economic Development Roundtable, October 10, 2019 Slide 7 – Population UF Bureau of Economic Research (BEBR) Southwest Florida Water Management District (SWFWMD) Pinellas County Metropolitan Planning Organization (MPO) Slide 8 – Population ESRI Business Analyst Online (BAO), 2019 Slide’s 23 to 30 – Employment US Census Bureau, 2017 Longitudinal Employer-Household Dynamics, OnTheMap Slide 31 – Target Industries St. Petersburg Economic and Workforce Development Department Slide 32 – Travel Time To Work US Census Bureau, 2013-2017 American Community Survey 5-year Estimates Slide’s 36 to 41 – Office Market Avision, Young Tampa Bay Office Report, Q3 2019 Statistics Slide 42 – Office Downtown Tenant CoStar, St. Petersburg City Directories Mix Slide 43 to 47 – Project Employment Moody’s 30-year forecast, Total No-Agricultural Employment, 2019 -

Assessment of the Cumulative Effects of Restoration Activities on Water Quality in Tampa Bay, Florida
Estuaries and Coasts https://doi.org/10.1007/s12237-019-00619-w MANAGEMENT APPLICATIONS Assessment of the Cumulative Effects of Restoration Activities on Water Quality in Tampa Bay, Florida Marcus W. Beck1 & Edward T. Sherwood2 & Jessica Renee Henkel3 & Kirsten Dorans4 & Kathryn Ireland5 & Patricia Varela6 Received: 10 April 2019 /Revised: 13 June 2019 /Accepted: 26 July 2019 # The Author(s) 2019 Abstract Habitat and water quality restoration projects are commonly used to enhance coastal resources or mitigate the negative impacts of water quality stressors. Significant resources have been expended for restoration projects, yet much less attention has focused on evaluating broad regional outcomes beyond site-specific assessments. This study presents an empirical framework to evaluate multiple datasets in the Tampa Bay area (Florida, USA) to identify (1) the types of restoration projects that have produced the greatest improvements in water quality and (2) time frames over which different projects may produce water quality benefits. Information on the location and date of completion of 887 restoration projects from 1971 to 2017 were spatially and temporally matched with water quality records at each of the 45 long-term monitoring stations in Tampa Bay. The underlying assumption was that the developed framework could identify differences in water quality changes between types of restoration projects based on aggregate estimates of chlorophyll-a concentrations before and after the completion of one to many projects. Water infra- structure projects to control point source nutrient loading into the Bay were associated with the highest likelihood of chlorophyll- a reduction, particularly for projects occurring prior to 1995. Habitat restoration projects were also associated with reductions in chlorophyll-a, although the likelihood of reductions from the cumulative effects of these projects were less than those from infrastructure improvements alone. -
Turkey Point Units 6 & 7 COLA
Turkey Point Units 6 & 7 COL Application Part 2 — FSAR SUBSECTION 2.4.1: HYDROLOGIC DESCRIPTION TABLE OF CONTENTS 2.4 HYDROLOGIC ENGINEERING ..................................................................2.4.1-1 2.4.1 HYDROLOGIC DESCRIPTION ............................................................2.4.1-1 2.4.1.1 Site and Facilities .....................................................................2.4.1-1 2.4.1.2 Hydrosphere .............................................................................2.4.1-3 2.4.1.3 References .............................................................................2.4.1-12 2.4.1-i Revision 6 Turkey Point Units 6 & 7 COL Application Part 2 — FSAR SUBSECTION 2.4.1 LIST OF TABLES Number Title 2.4.1-201 East Miami-Dade County Drainage Subbasin Areas and Outfall Structures 2.4.1-202 Summary of Data Records for Gage Stations at S-197, S-20, S-21A, and S-21 Flow Control Structures 2.4.1-203 Monthly Mean Flows at the Canal C-111 Structure S-197 2.4.1-204 Monthly Mean Water Level at the Canal C-111 Structure S-197 (Headwater) 2.4.1-205 Monthly Mean Flows in the Canal L-31E at Structure S-20 2.4.1-206 Monthly Mean Water Levels in the Canal L-31E at Structure S-20 (Headwaters) 2.4.1-207 Monthly Mean Flows in the Princeton Canal at Structure S-21A 2.4.1-208 Monthly Mean Water Levels in the Princeton Canal at Structure S-21A (Headwaters) 2.4.1-209 Monthly Mean Flows in the Black Creek Canal at Structure S-21 2.4.1-210 Monthly Mean Water Levels in the Black Creek Canal at Structure S-21 2.4.1-211 NOAA -

Supporting Information for Canal Evaluations
Restoration Strategies Regional Water Quality Plan – Science Plan for the Everglades Stormwater Treatment Areas: Evaluation of the Influence of Canal Conveyance Features on STA and FEB Inflow and Outflow TP Concentrations Supporting Information for Canal Evaluations WR-2015-003 Prepared by: Hongying Zhao, Ph.D., P.E., Tracey Piccone, P.E., and Orlando Diaz, Ph.D. South Florida Water Management District and Tetra Tech, Inc. 759 South Federal Highway, Suite 314 Stuart, FL 34994 July 2015 Revised September 16, 2015 Restoration Strategies Science Plan - Evaluation of the Influence of Canal Conveyance Features on STA and FEB Inflow and Outflow TP Concentrations – Supporting Information for Canal Evaluations Acknowledgments The authors thank Delia Ivanoff, Kim O’Dell, and Larry Schwartz for support throughout this study; Jeremy McBryan, Larry Gerry, Seán Sculley, and Ceyda Polatel for support in developing and reviewing the Detailed Study Plan; Michael Chimney, Wossenu Abtew, Larry Schwartz, and Seán Sculley for reviewing the early draft; and Stacey Ollis for detailed editing of this technical report. 2 Restoration Strategies Science Plan - Evaluation of the Influence of Canal Conveyance Features on STA and FEB Inflow and Outflow TP Concentrations – Supporting Information for Canal Evaluations TABLE OF CONTENTS Part I: Literature Review .............................................................................................................................. 5 Transport .................................................................................................................................................. -

Draft Sea Level Rise Methodology Recommendation.Docx
RECOMMENDED PROJECTIONS OF SEA LEVEL RISE IN THE TAMPA BAY REGION Tampa Bay Climate Science Advisory Panel Updated April 2019 RECOMMENDED PROJECTIONS OF SEA LEVEL RISE IN THE TAMPA BAY REGION Executive Summary In this document, the Tampa Bay Climate Science Advisory Panel (CSAP) recommends a common set of sea level rise (SLR) projections for use throughout the Tampa Bay region. The recommendation establishes the foundation for a coordinated approach to address the effects of a changing climate, which advances the objectives of the newly-established Tampa Bay Regional Resiliency Coalition. Local governments and other agencies planning for SLR in the Tampa Bay region should incorporate the following key findings of this CSAP recommendation. • Data measured at the St. Petersburg tide gauge shows that water levels in Tampa Bay have already increased approximately 7.8 inches since 1946. • Based upon a thorough assessment of scientific data and literature, the Tampa Bay region can expect to see an additional 2 to 8.5 feet of SLR by 2100. • Projections of SLR should be consistent with present and future National Climate Assessment estimates and methods. The NOAA Low scenario should not be used for planning purposes. • Projections of SLR should be regionally corrected using St. Petersburg tide gauge data. • Adaptation planning should employ a scenario-based approach that, at minimum, considers location, time horizon, and risk tolerance. Introduction Formed in spring 2014, the CSAP is an ad hoc network of scientists and resource managers working in the Tampa Bay region (Figure 1). The group’s goal is to collaboratively develop science-based recommendations for local governments and regional agencies as they respond to climate change, including associated sea level change.