Scale Insects, Mealybugs, Whiteflies and Psyllids (Hemiptera
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ladybirds, Ladybird Beetles, Lady Beetles, Ladybugs of Florida, Coleoptera: Coccinellidae1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. EENY-170 Ladybirds, Ladybird beetles, Lady Beetles, Ladybugs of Florida, Coleoptera: Coccinellidae1 J. H. Frank R. F. Mizell, III2 Introduction Ladybird is a name that has been used in England for more than 600 years for the European beetle Coccinella septempunctata. As knowledge about insects increased, the name became extended to all its relatives, members of the beetle family Coccinellidae. Of course these insects are not birds, but butterflies are not flies, nor are dragonflies, stoneflies, mayflies, and fireflies, which all are true common names in folklore, not invented names. The lady for whom they were named was "the Virgin Mary," and common names in other European languages have the same association (the German name Marienkafer translates Figure 1. Adult Coccinella septempunctata Linnaeus, the to "Marybeetle" or ladybeetle). Prose and poetry sevenspotted lady beetle. Credits: James Castner, University of Florida mention ladybird, perhaps the most familiar in English being the children's rhyme: Now, the word ladybird applies to a whole Ladybird, ladybird, fly away home, family of beetles, Coccinellidae or ladybirds, not just Your house is on fire, your children all gone... Coccinella septempunctata. We can but hope that newspaper writers will desist from generalizing them In the USA, the name ladybird was popularly all as "the ladybird" and thus deluding the public into americanized to ladybug, although these insects are believing that there is only one species. There are beetles (Coleoptera), not bugs (Hemiptera). many species of ladybirds, just as there are of birds, and the word "variety" (frequently use by newspaper 1. -

Arthropods of Elm Fork Preserve
Arthropods of Elm Fork Preserve Arthropods are characterized by having jointed limbs and exoskeletons. They include a diverse assortment of creatures: Insects, spiders, crustaceans (crayfish, crabs, pill bugs), centipedes and millipedes among others. Column Headings Scientific Name: The phenomenal diversity of arthropods, creates numerous difficulties in the determination of species. Positive identification is often achieved only by specialists using obscure monographs to ‘key out’ a species by examining microscopic differences in anatomy. For our purposes in this survey of the fauna, classification at a lower level of resolution still yields valuable information. For instance, knowing that ant lions belong to the Family, Myrmeleontidae, allows us to quickly look them up on the Internet and be confident we are not being fooled by a common name that may also apply to some other, unrelated something. With the Family name firmly in hand, we may explore the natural history of ant lions without needing to know exactly which species we are viewing. In some instances identification is only readily available at an even higher ranking such as Class. Millipedes are in the Class Diplopoda. There are many Orders (O) of millipedes and they are not easily differentiated so this entry is best left at the rank of Class. A great deal of taxonomic reorganization has been occurring lately with advances in DNA analysis pointing out underlying connections and differences that were previously unrealized. For this reason, all other rankings aside from Family, Genus and Species have been omitted from the interior of the tables since many of these ranks are in a state of flux. -

Biological Control of Hemlock Woolly Adelgid
Forest Health Technology Enterprise Team TECHNOLOGY TRANSFER Biological Control BIOLOGICAL CONTROL OF HEMLOCK WOOLLY ADELGID TECHNICALCONTRIBUTORS: RICHARD REARDON FOREST HEALTH TECHNOLOGY ENTERPRISE TEAM, USDA FOREST SERVICE, MORGANTOWN, WEST VIRGINIA BRAD ONKEN FOREST HEALTH PROTECTION, USDA FOREST SERVICE, MORGANTOWN, WEST VIRGINIA AUTHORS: CAROLE CHEAH THE CONNECTICUT AGRICULTURAL EXPERIMENT STATION MIKE MONTGOMERY NORTHEASTERN RESEARCH STATION SCOTT SALEM VIRGINIA POLYTECHNIC INSTITUTE AND STATE UNIVERSITY BRUCE PARKER, MARGARET SKINNER, SCOTT COSTA UNIVERSITY OF VERMONT FHTET-2004-04 U.S. Department Forest of Agriculture Service FHTET he Forest Health Technology Enterprise Team (FHTET) was created in T1995 by the Deputy Chief for State and Private Forestry, USDA, Forest Service, to develop and deliver technologies to protect and improve the health of American forests. This book was published by FHTET as part of the technology transfer series. http://www.fs.fed.us/foresthealth/technology/ On the cover Clockwise from top left: adult coccinellids Sasajiscymnus tsugae, Symnus ningshanensis, and Scymnus sinuanodulus, adult derodontid Laricobius nigrinus, hemlock woolly adelgid infected with Verticillium lecanii. For copies of this publication, please contact: Brad Onken Richard Reardon Forest Health Protection Forest Health Technology Enterprise Morgantown, West Virginia Team Morgantown, West Virginia 304-285-1546 304-285-1566 [email protected] [email protected] All images in the publication are available online at http://www.forestryimages.org and http://www.invasive.org Reference numbers for the digital files appear in the figure captions in this publication. The entire publication is available online at http://www.bugwood.org and http://www.fs.fed.us/na/morgantown/fhp/hwa. The U.S. -

Coleoptera: Coccinellidae): Influence of Subelytral Ultrastructure
Experimental & Applied Acarology, 23 (1999) 97–118 Review Phoresy by Hemisarcoptes (Acari: Hemisarcoptidae) on Chilocorus (Coleoptera: Coccinellidae): influence of subelytral ultrastructure M.A. Houck* Department of Biological Sciences, Texas Tech University, Lubbock, TX 79409–3131, USA (Received 9 January 1997; accepted 17 April 1998) ABSTRACT The non-phoretic stages of mites of the genus Hemisarcoptes are predators of the family Diaspididae. The heteromorphic deutonymph (hypopus) maintains a stenoxenic relationship with beetles of the genus Chilocorus. The mites attach to the subelytral surface of the beetle elytron during transport. There is variation in mite density among species of Chilocorus. Both Hemisarcoptes and Chilocorus have been applied to biological control programmes around the world. The objective of this study was to determine whether subelytral ultrastructure (spine density) plays a role in the evolution of symbiosis between the mite and the beetle. The subelytral surfaces of 19 species of Chilocorus and 16 species of Exochomus were examined. Spine density was determined for five subelytral zones: the anterior pronotal margin, medial central region, caudoventral tip, lateral distal margin and epipleural region. Spine density on the subelytral surface of Chilocorus and Exochomus was inversely correlated with the size of the elytron for all zones except the caudoventral tip. This suggests that an increase in body size resulted in a redistribution of spines and not an addition of spines. The pattern of spine density in Exochomus and Chilocorus follows a single size–density trajectory. The pattern of subelytral ultrastructure is not strictly consistent with either beetle phylogeny or beetle allometry. The absence of spines is not correlated with either beetle genus or size and species of either Chilocorus or Exochomus may be devoid of spines in any zone, irrespective of body size. -
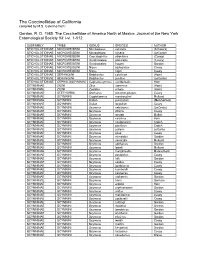
The Coccinellidae of California Compiled by M.S
The Coccinellidae of California compiled by M.S. Caterino from: Gordon, R. D. 1985. The Coccinellidae of America North of Mexico. Journal of the New York Entomological Society 93: i-vi, 1-912. SUBFAMILY TRIBE GENUS SPECIES AUTHOR STICHOLOTIDINAE MICROWEISEINI Microweisea suturalis (Schwarz) STICHOLOTIDINAE MICROWEISEINI Microweisea misella (LeConte) STICHOLOTIDINAE MICROWEISEINI Coccidophilus atronitens (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea planiceps (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea hageni Gordon STICHOLOTIDINAE MICROWEISEINI Nipus biplagiatus Casey STICHOLOTIDINAE MICROWEISEINI Nipus niger Casey STICHOLOTIDINAE SERANGIINI Delphastus catalinae (Horn) STICHOLOTIDINAE SERANGIINI Delphastus pusillus (LeConte) STICHOLOTIDINAE CEPHALOSCYMNINI Cephaloscymnus occidentalis Horn SCYMNINAE ZILINI Zilus aterrimus (Horn) SCYMNINAE ZILINI Zagloba ornata (Horn) SCYMNINAE STETHORINI Stethorus punctum picipes Casey SCYMNINAE SCYMNINI Cryptolaemus montrouzieri Mulsant SCYMNINAE SCYMNINI Didion punctatum (Melsheimer) SCYMNINAE SCYMNINI Didion longulum Casey SCYMNINAE SCYMNINI Scymnus nebulosus (LeConte) SCYMNINAE SCYMNINI Scymnus dificilis Casey SCYMNINAE SCYMNINI Scymnus fenderi Malkin SCYMNINAE SCYMNINI Scymnus caurinus Horn SCYMNINAE SCYMNINI Scymnus coniferarum Crotch SCYMNINAE SCYMNINI Scymnus pacificus Crotch SCYMNINAE SCYMNINI Scymnus pallens LeConte SCYMNINAE SCYMNINI Scymnus gilae Casey SCYMNINAE SCYMNINI Scymnus mimoides Gordon SCYMNINAE SCYMNINI Scymnus cervicalis Mulsant SCYMNINAE SCYMNINI Scymnus apithanus Gordon -

Citrus Mealybug Planococcus Citri (Risso) (Insecta: Hemiptera: Pseudococcidae)1 Harsimran Kaur Gill, Gaurav Goyal, and Jennifer Gillett-Kaufman2
EENY-537 Citrus Mealybug Planococcus citri (Risso) (Insecta: Hemiptera: Pseudococcidae)1 Harsimran Kaur Gill, Gaurav Goyal, and Jennifer Gillett-Kaufman2 Introduction or several weeks. An average of 29 eggs per day is laid by females (Kerns et al. 2001, Meyers 1932). The citrus mealybug is a common pest of citrus primarily in greenhouses and of several ornamental plants in Florida. It has been recognized as a difficult-to-control pest in Europe since 1813 (where it is called the greenhouse mealybug) and in the United States since 1879 (Anonymous 2007). Distribution The pest is a native of Asia but is also found throughout the Americas, Europe, and Oceania. In North America, it is present in both Mexico and United States (Alabama, Arizona, Arkansas, California, Florida, Hawaii, Kansas, Louisiana, Maryland, Massachusetts, Missouri, New Mexico, Ohio, South Carolina, Tennessee, Texas, and Virginia) (CABI/EPPO 1999). Figure 1. Eggs are deposited as white cottony massess called ovisacs. Credits: Lyle Buss, University of Florida. Description and Life History Immatures Eggs Nymphs emerge from the ovisacs and typically settle along Eggs are deposited as white, cottony masses, called ovisacs, midribs and veins on the underside of leaves, young twigs, on the trunk and stems of citrus plants, giving the appear- and fruit buttons. They can also be found where two fruits ance of cotton spread on plants (Figure 1). The glossy, are touching each other (Figure 2) or on leaves clinging light yellow eggs are oval and approximately 0.3 mm long. to fruits. Due to their habit of hiding in crevices, light A female can lay from 300 to 600 eggs in her life period, infestations are easily overlooked. -

Crapemyrtle Bark Scale Acanthococcus Lagerstroemiae Kuwana (Hemiptera: Eriococcidae): Analysis of Factors Influencing Infestation and Control
CRAPEMYRTLE BARK SCALE ACANTHOCOCCUS LAGERSTROEMIAE KUWANA (HEMIPTERA: ERIOCOCCIDAE): ANALYSIS OF FACTORS INFLUENCING INFESTATION AND CONTROL A Thesis by KYLE ANDREW GILDER Submitted to the Office of Graduate and Professional Studies of Texas A&M University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Chair of Committee, Kevin M. Heinz Co-Chair of Committee, Mengmeng Gu Committee Members, Mike Merchant Head of Department, Phillip Kaufman December 2020 Major Subject: Entomology Copyright 2020 Kyle Andrew Gilder ABSTRACT Crapemyrtle bark scale, Acanthococcus lagerstroemiae (Kuwana), a new non-native pest from Asia first discovered in the U.S. in 2004 has now been reported in 14 states. The scale jeopardizes the future of crapemyrtles use as a popular ornamental landscape tree in the U.S. Management of this pest will likely include biological strategies. Before such strategies can be implemented it is important to examine relative abundances and distributions of arthropod species associated with the scale in the geographic area targeted for biological control. In the first objective, surveys of crapemyrtle ecology from two varietal groups of crapemyrtle trees (Lagerstroemia spp.) were undertaken in Tarrant and Brazos counties across six consecutive seasons in 2018 – 2019. A rich arthropod community was discovered. The most common predators were spiders, coccinellids, and chrysopids. Insects in the families Eriococcidae, Aphididae, and Thripidae were common herbivores on Lagerstroemia spp. Numerous phytophagous and mycophagous mites were also collected. These herbivores constitute a reservoir of alternative prey for generalist predators that may also feed on A. lagerstroemiae. A food web was constructed to illustrate direct and indirect effects of the predator community on A. -

Beneficial Insects of Utah Guide
BENEFICIAL INSECTS OF UTAH beneficial insects & other natural enemies identification guide PUBLICATION COORDINATORS AND EDITORS Cami Cannon (Vegetable IPM Associate and Graphic Design) Marion Murray (IPM Project Leader) AUTHORS Cami Cannon Marion Murray Ron Patterson (insects: ambush bug, collops beetle, red velvet mite) Katie Wagner (insects: Trichogramma wasp) IMAGE CREDITS All images are provided by Utah State University Extension unless otherwise noted within the image caption. CONTACT INFORMATION Utah State University IPM Program Dept. of Biology 5305 Old Main Hill Logan, UT 84322 (435) 797-0776 utahpests.usu.edu/IPM FUNDING FOR THIS PUBLICATION WAS PROVIDED BY: USU Extension Grants Program CONTENTS PREFACE Purpose of this Guide ................................................................6 Importance of Natural Enemies ..................................................6 General Practices to Enhance Natural Enemies ...........................7 Plants that will Enhance Natural Enemy Populations ..................7 PREDATORS Beetles .....................................................................................10 Flies .........................................................................................24 Lacewings/Dustywings .............................................................32 Mites ........................................................................................36 Spiders .....................................................................................42 Thrips ......................................................................................44 -

A Guide to Scale Insect Identification1 S
HS-817 A Guide to Scale Insect Identification1 S. H. Futch, C. W. McCoy, and C. C. Childers2 Control of scale insects in Florida citrus utilizes native and maximum suppression of scales, the young mobile nymphal introduced exotic natural enemies, including predators, stages, referred to as crawlers, should be targeted as they parasites, and pathogens. Under most conditions, predators are more susceptible to control than the eggs, settled larger and parasites suppress scale pest populations to a level nymphal, or adult stages of the scales. where chemical intervention is unnecessary. In situations where natural enemies do not provide the necessary Armored Scale control, integrated pest management (IPM) is used since it minimizes negative effects on natural enemies. Increases in Purple Scale–(Newman) scale insect populations involve multiple factors including: The armor of the adult female scale is 2 to 3 mm long, a) disruption of biological control by weather; b) infestation purple to dark brown, elongated, and usually curved (Fig. of areas by scale insects where natural enemies do not exist; 1). The armor of the male is much shorter and more slender and c) disruption of natural enemies by the repeated use of than the female. The eggs are found under the scale cover non-selective pesticides. and after hatching, the pearl white crawlers are less than 0.25 mm in length. Infestations may occur at any time, but When scale insects are not controlled by biological or are usually highest during the spring. Purple scales prefer chemical means, high populations damage leaves, fruit, a tree with a dense canopy and are found on leaves, twigs, twigs, branches, and/or tree trunks. -

(Coleoptera; Coccinellidae), from a MITE, Hemisarcoptes Cooremani
THE UPTAKE OF TRITIATED WATER BY A BEETLE, Chilocorus cacti (Coleoptera; Coccinellidae), FROM A MITE, Hemisarcoptes cooremani (Acari: Acariformes) by AURAL! E. HOLTE, B.S. A THESIS IN BIOLOGY Submitted to the Graduate Faculty of Texas Tech University in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Approved December, 1999 mr'-^'^-"""'^'" .——^—--— •« • "ji» • »j»«p»^"^i^»w /^n^^l C>^i^f ACKNOWLEDGMENTS I thank a number of people for their assistance and support in the completion of iS-f' 7^ this work. First, I would like to thank Dr. Marilyn Houck for her generous encouragement, understanding and guidance, without which I would not have been able to start or complete this project. I also thank the members of my committee. Dr. Nathan Collie and Dr. Richard Deslippe who provided valuable comments and information utilized for this research. Elizabeth Richards, Heather Roberts, and Qingtian Li were encouraging and helpful colleagues in all my endeavors as a graduate student. I also thank a number of people for personal support; foremost, Damon for without his devoted love, constant support and immutable encouragement, I would have not been able to accomplish this work. I v^sh to thank my family for all of the love and understanding they have given me, especially my mother who guided me with her example, demonstrating that I could do anything once I set my mind to it. I also would like to acknowledge all of the other friends and family members who have given me encouragement. Finally, financial support for this research was provided by the Texas Tech University Biology Department and the Bi-National Agricultural Research and Development grants (#IS-1397-87 and #US-2359-93C to M. -

Coleoptera:Coccinellidae) Associated with Major Sucking Pests of Kerala
Journal of Biological Control, 31(4): 212-216, 2017, DOI: 10.18311/jbc/2017/18618 Research Article Scymnini (Coleoptera:Coccinellidae) associated with major sucking pests of Kerala C. V. VIDYA and HASEENA BHASKAR* Department of Agricultural Entomology, College of Horticulture, Kerala Agricultural University, Thrissur - 680656, Kerala, India *Corresponding author E-mail: [email protected] ABSTRACT: An extensive survey was undertaken to explore the diversity of fauna of Scymnini associated with sucking pests viz., mealybugs, aphids and whiteflies on fruits, vegetables, plantation crops, ornamentals and other associated plants across Kerala during 2015-17. Scymnini along with associated prey were collected, and in the laboratory, beetles were dissected to study the male genitalia for identification, while prey were identified by the expert in the concerned taxa at NBAIR. The study recorded 14 species of Scymnini in five genera associated with 19 species of prey (12 mealybugs, 5 aphids and 2 whiteflies). The five genera include Axinoscymnus, Cryp- tolaemus, Horniolus, Nephus and Scymnus. Scymnus, the predominant genus was represented by three subgenera viz., S. (Scymnus), S. (Pullus) and S. (Neopullus). S.(Pullus) coccivora Ayyar recorded the maximum prey range of six species of mealy bugs. Nephus regularis (Sicard) was recorded for the first time in Kerala. The study identified Toxoptera odinae (van der Goot) as a new prey record for S. (P.) pyrocheilus Mulsant. KEY WORDS: Axinoscymnus, Cryptolaemus, Horniolus, Nephus, Predatory Coccinellids, Scymnini, Scymnus, Sucking Pests (Article chronicle: Received: 02-09-2017; Revised: 15-11-2017; Accepted: 12-12-2017) INTRODUCTION in agricultural ecosystems of Kerala. Hence the present study was undertaken to record the species composition of Coccinellid beetles always attracted the attention of Scymnini associated with major sucking pests in different biocontrol scientists and have been exploited as predators crop plants of Kerala. -
An Annotated Checklist of Ladybeetle Species (Coleoptera, Coccinellidae) of Portugal, Including the Azores and Madeira Archipelagos
ZooKeys 1053: 107–144 (2021) A peer-reviewed open-access journal doi: 10.3897/zookeys.1053.64268 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research An annotated checklist of ladybeetle species (Coleoptera, Coccinellidae) of Portugal, including the Azores and Madeira Archipelagos António Onofre Soares1, Hugo Renato Calado2, José Carlos Franco3, António Franquinho Aguiar4, Miguel M. Andrade5, Vera Zina3, Olga M.C.C. Ameixa6, Isabel Borges1, Alexandra Magro7,8 1 Centre for Ecology, Evolution and Environmental Changes and Azorean Biodiversity Group, Faculty of Sci- ences and Technology, University of the Azores, 9500-321, Ponta Delgada, Portugal 2 Azorean Biodiversity Group, Faculty of Sciences and Technology, University of the Azores, 9501-801, Ponta Delgada, Portugal 3 Centro de Estudos Florestais (CEF), Instituto Superior de Agronomia, Universidade de Lisboa, 1349-017, Lisboa, Portugal 4 Laboratório de Qualidade Agrícola, Caminho Municipal dos Caboucos, 61, 9135-372, Camacha, Madeira, Portugal 5 Rua das Virtudes, Barreiros Golden I, Bloco I, R/C B, 9000-645, Funchal, Madeira, Portugal 6 Centre for Environmental and Marine Studies and Department of Biology, University of Aveiro, Campus Universitário de Santiago, 3810-193, Aveiro, Portugal 7 Laboratoire Evolution et Diversité biologique, UMR 5174 CNRS, UPS, IRD, 118 rt de Narbonne Bt 4R1, 31062, Toulouse cedex 9, France 8 University of Toulouse – ENSFEA, 2 rt de Narbonne, Castanet-Tolosan, France Corresponding author: António Onofre Soares ([email protected]) Academic editor: J. Poorani | Received 10 February 2021 | Accepted 24 April 2021 | Published 2 August 2021 http://zoobank.org/79A20426-803E-47D6-A5F9-65C696A2E386 Citation: Soares AO, Calado HR, Franco JC, Aguiar AF, Andrade MM, Zina V, Ameixa OMCC, Borges I, Magro A (2021) An annotated checklist of ladybeetle species (Coleoptera, Coccinellidae) of Portugal, including the Azores and Madeira Archipelagos.