Introduction to Water Desalination
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Making Decisions About Water and Wastewater for Aqueous Operation
Making Decisions about Water and Wastewater for Aqueous Operation John F. Russo Chapter 2.17 Handbook for Critical Cleaning Editor-in-Chief Barbara Kanegsberg Reprinted with permission from CRC Press www.crcpress.com INTRODUCTION..................................................................................................................................3 TYPICAL CLEANING SYSTEM............................................................................................................3 OPERATIONAL SITUATIONS OF TYPICAL USER ...............................................................................4 Determining the Water Purity Requirements .........................................................................................4 Undissolved Contaminants............................................................................................................4 Dissolved Contaminants...............................................................................................................4 Undissolved and Dissolved Contaminants........................................................................................5 Other Conditions...........................................................................................................................5 Determining the Wastewater Volume Produced .....................................................................................6 Source Water Trea tment .....................................................................................................................6 No -

Industrial Experiment on Electrodialized Separation of Highly Concentrated Multicomponent Technological Solutions at Thermal Power Plants
E3S Web of Conferences 124, 01029 (2019) https://doi.org/10.1051/e3sconf/201912401029 SES-2019 Industrial experiment on electrodialized separation of highly concentrated multicomponent technological solutions at thermal power plants A. A. Filimonova1, N. D. Chichirova1, A. A. Chichirov1,*, and A. I. Minibaev1 1 Kazan State Power Engineering University, Kazan, Russia Abstract. The main sources of highly concentrated multicomponent technological solutions at thermal power plants (TPPs) are water treatment plants. Analysis of operation of the ion-exchange water treatment plant at the Nizhnekamsk Thermal Power Plant-1 showed that half of alkali supplied to regeneration of the anion-exchange alkali filters is not used, but is discharged for neutralization and then to wastewater. Due to the fact that the cost of alkali used in technological processes is quite high, it is economically feasible to process the alkaline waste with the alkali extraction and its reuse in the production cycle. The article presents the experimental results on the electro-membrane separation of alkaline waste regeneration solutions and wash water after anion-exchange filter regeneration. The revealed differences in the selectivity of various ion transfer through the electrodialysis apparatus membranes, depending on time and amount of transmitted electricity, allowed us to establish the possibility of obtaining an alkaline solution purified from impurities. 1 Introduction biotechnology, pharmaceuticals, water treatment at power plants, wastewater treatment [12–16]. Thermal power plants (TPPs) in Russia use The main advantage of electrochemical, and electrochemical methods of water treatment in a small especially electro-membrane methods, is that chemical volume and only as additional methods of water reactions and transformations are conducted using purification [1–4]. -

Ion Exchange and Disposal Issues Associated with the Brine Waste Stream
FWRJ Ion Exchange and Disposal Issues Associated With the Brine Waste Stream Julie Karleskint, Daniel Schmidt, Robert Anderson, Jayson Page, and A.J. Berndt he City of Arcadia recently completed from the local water supply authority, it was Julie Karleskint, P.E., is a senior construction of a new 1.5-mil-gal-per- determined that ion exchange would be the associate with Hazen and Sawyer in Tday (mgd) water treatment plant most cost-effective option for construction. A Sarasota. Daniel Schmidt, P.E., is a (WTP) using ion exchange technology to re- reduction in capacity was also provided since senior associate with Hazen and Sawyer place its 3-mgd lime softening WTP. The lime the City’s water supply source, groundwater in Tampa. Robert Anderson, P.E., is a plant had reached the end of its serviceable life from the intermediate aquifer, was limited senior associate with Hazen and Sawyer and the treatment of groundwater for the re- based on current pumping limitations and in Orlando. Jayson Page, P.E., is a moval of radionuclides, hardness, sulfides, or- permitted capacity. senior associate with Hazen and Sawyer ganic carbon, and fluoride was desired in The groundwater is supplied from six order to provide safe drinking water to the wells, approximately 350 ft deep and located in Coral Gables. A.J. Berndt is the utility community. After evaluating several treatment within a 1-mi radius of the plant. A summary director for the City of Arcadia. technologies, including lime treatment, of the water quality from the wellfield is shown nanofiltration, ion exchange, and purchases in Table 1. -

Review of the Ion Exchange Filtration Process and Materials Used August 4, 2020
National Organic Standards Board Handli ng Subcommittee Proposal Review of the Ion Exchange Filtration Process and Materials Used August 4, 2020 Background: In an August 27, 2019, memo the National Organic Program requested the NOSB provide recommendations related to the process of ion exchange filtration in the handling of organic products. It has become clear that there is inconsistency between certifiers in how they approve or disapprove this type of process. Some certifiers require only the solutions that are used to recharge the ion exchange membranes be on the National List at § 205.605. Others require that all materials, including ion exchange membranes and resins be on the National List. The National Organic Program provided clarification to certifying agents in an email sent on May 7, 2019, that nonagricultural substances used in the ion-exchange process must be present on the National List. This would include, but is not limited to, resins, membranes and recharge materials. Originally, the NOP asked all operations to come into compliance with the statement above by May 1, 2020. However, in response to requests for clarification of NOP’s rationale, as well as requests to extend the timeline for implementation, the NOP delayed the implementation date in order to gather more information and requested that NOSB review the issue. Manufacturers and certifiers who wish to continue allowance of the ion exchange process disagree with some of the findings of the NOP on this complex issue. The different opinions of the need for resins, recharge materials and membranes to be present on the National List, as well as how they interact with each other and the liquid run through the process, is complicated and the NOP therefore asked the NOSB to take on this issue. -

Mass Transfer Phenomena During Electrodialysis of Multivalent Ions: Chemical Equilibria and Overlimiting Currents
applied sciences Article Mass Transfer Phenomena during Electrodialysis of Multivalent Ions: Chemical Equilibria and Overlimiting Currents Manuel César Martí-Calatayud * , Montserrat García-Gabaldón and Valentín Pérez-Herranz * IEC Group, Departament d’Enginyeria Quimica i Nuclear, Universitat Politècnica de València, Camí de Vera s/n, 46022 València, Spain; [email protected] * Correspondence: [email protected] (M.C.M.-C.); [email protected] (V.P.-H.); Tel.: +34-96-3877632 (V.P.-H.) Received: 26 July 2018; Accepted: 3 September 2018; Published: 6 September 2018 Featured Application: Selective ion transport through polymer electrolytes is crucial for environmental applications, especially in deionization of water for drinking and irrigation purposes and in effluents’ treatment. Ion transport through permselective membranes is relevant in emerging energy applications as well. Abstract: Electrodialysis is utilized for the deionization of saline streams, usually formed by strong electrolytes. Recently, interest in new applications involving the transport of weak electrolytes through ion-exchange membranes has increased. Clear examples of such applications are the recovery of valuable metal ions from industrial effluents, such as electronic wastes or mining industries. Weak electrolytes give rise to a variety of ions with different valence, charge sign and transport properties. Moreover, development of concentration polarization under the application of an electric field promotes changes in the chemical equilibrium, thus making more complex understanding of mass transfer phenomena in such systems. This investigation presents a set of experiments conducted with salts of multivalent metals with the aim to provide better understanding on the involved mass transfer phenomena. Chronopotentiometric experiments and current-voltage characteristics confirm that shifts in chemical equilibria can take place simultaneous to the activation of overlimiting mass transfer mechanisms, that is, electroconvection and water dissociation. -

Commercial Thermal Technologies for Desalination of Water from Renewable Energies: a State of the Art Review
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 4 January 2021 doi:10.20944/preprints202101.0033.v1 Review Commercial Thermal Technologies for Desalination of Water from Renewable Energies: A State of the Art Review Jhon Feria-Díaz 1, 2, *, María López-Méndez 1, Juan Rodríguez-Miranda 3, Luis Sandoval-Herazo 1 and Felipe Correa-Mahecha 4 1 Instituto Tecnológico Superior de Misantla, Km 1.8 Carretera Lomas del Cojolite, 93821 Misantla, México; [email protected]; [email protected]; [email protected] 2 Universidad de Sucre, Cra. 28 #5-267, Sincelejo, Colombia; [email protected] 3 Universidad Distrital Francisco José de Caldas, Cra. 7 #40b-53, Bogotá, Colombia; [email protected] 4 Fundación Universidad de América, Avda Circunvalar No. 20-53, Bogotá, Colombia; [email protected] * Correspondence: [email protected] Abstract: Thermal desalination is yet a reliable technology in the treatment of brackish water and seawater; however, its demanding high energy requirements have lagged it compared to other non- thermal technologies such as reverse osmosis. This review provides an outline of the development and trends of the three most commercially used thermal or phase change technologies worldwide: Multi Effect Distillation (MED), Multi Stage Flash (MSF), and Vapor Compression Distillation (VCD). First, state of water stress suffered by regions with little fresh water availability and existing desalination technologies that could become an alternative solution are shown. The most recent studies published for each commercial thermal technology are presented, focusing on optimizing the desalination process, improving efficiencies, and reducing energy demands. Then, an overview of the use of renewable energy and its potential for integration into both commercial and non- commercial desalination systems is shown. -

Reverse Osmosis As a Pretreatment of Ion Exchange Equipment at PSE
Copyright Warning & Restrictions The copyright law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be “used for any purpose other than private study, scholarship, or research.” If a, user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use” that user may be liable for copyright infringement, This institution reserves the right to refuse to accept a copying order if, in its judgment, fulfillment of the order would involve violation of copyright law. Please Note: The author retains the copyright while the New Jersey Institute of Technology reserves the right to distribute this thesis or dissertation Printing note: If you do not wish to print this page, then select “Pages from: first page # to: last page #” on the print dialog screen The Van Houten library has removed some of the personal information and all signatures from the approval page and biographical sketches of theses and dissertations in order to protect the identity of NJIT graduates and faculty. ABSTRACT REVERSE OSMOSIS AS A PRETREATMENT FOR ION EXCHANGE AT PSE&G'S HUDSON GENERATING STATION by Steven Leon Public Service Electric and Gas Company's Hudson Generating Station has historically had problems providing sufficient high quality water for its two once through, supercritical design boilers. The station requires over 60 million gallons annually to compensate for system losses. -

Electrodialysis Principle
Setup of a 20 m3/h ED/RO plant to produce pure water from river water. A case study focusing on the electrodialysis process and the compatibility with RO pretreatment by Rudolf E. Brunner and Dr. Patrick Altmeier Ioncontract GmbH PCCell GmbH Znaimer Straße 34 Lebacher Straße 60 71263 Weil der Stadt 66265 Heusweiler [email protected] www.electrodialysis.de Electrodialysis principle • Anions move towards anode • Cations move towards cathode • Cation exchange membranes let cations through and block anions • Anion exchange membranes let anions go through and block cations • Electroneutrality 1 Electrodialysis Model Principle of electrodialysis is a stack of alternating cation and anion exchange membranes. Model: A tower block with alternating red and blue floors, filled with people Looking down, youmay see either blue or red floors. Electrodialysis Rules • Yellow: go up! Do not pass blue ceiling! • Green: go down! Do not pass red floor! 2 Apply Rules • All have moved until the blocking rule apply. • Result is: blocking rule apply in each second floor. • Note: We ignored the electroneutrality, for instance. An ED stack scheme 3 Continuous ED processing • A Diluate enter the cell, DC will be processed and Diluate Electrodialyzer out leave the cell as the finished product. Concen- trate out • The solute for the uptake of the ions enter the cell and leave it as the final concentrate. Electrode rinse Diluate • Electrode rinse will be in circulated (option: use of concentrate stream) Concen- trate in continuous mode Batch ED process DC • Each process solutionis Electrodialyzer hydraulical sealand cirulated often. • Ionic concentration shift slowly; each solution remain the same. -

Boron Removal from Dual-Staged Seawater Nanofiltration Permeate by Electrodialysis
Desalination and Water Treatment 10 (2009) 60–63 www.deswater.com October 1944-3994 / 1944-3986 © 2009 Desalination Publications. All rights reserved. doi: 10.5004/dwt.2009.782 Boron removal from dual-staged seawater nanofiltration permeate by electrodialysis Marian Turek*, Piotr Dydo, Barbara Bandura-Zalska Silesian University of Technology, Faculty of Chemistry, ul. B. Krzywoustego 6, 44–100 Gliwice, Poland Tel. +48 (32) 2372735; Fax +48 (32) 2372277; email: [email protected] Received 30 September 2008; accepted in revised form 10 July 2009 abstract The dual-staged nanofiltration to desalinate seawater is being proposed. The promising energy consumption, much lower than for RO seawater desalination, is reported. However, further reduc- tion in boron is needed. In the authors’ opinion, since the salinity of the second stage NF permeate is rather low, the easiest way to remove boron is to transfer it through an ion-exchange membrane (electrodialysis, ED). The relatively deep demineralization necessity is a shortcoming in the boron removal electrodialysis process, but ED seems to be privileged, since under these conditions boron (most likely borate) with its low mobility has to compete with small Cl– content only. In order to determine the applicability of the electrodialysis for boron removal from dual-staged nanofiltration the set of laboratory measurements was conducted. The simulated dual-staged nanofiltration per- 2+ 2+ + – 2– meate composition was as follows (mg/L): Mg — 0.2; Ca — 0.1; Na — 92; Cl — 117; SO4 — 0.2; B — 2.4. An ED unit, equipped with AMX and CMX Neosepta (Tokuyama Co.) membranes and 0.4 mm membrane-to-membrane distance, was applied. -

Wastewater Ion Exchange Services
WASTEWATER ION EXCHANGE SERVICES Evoqua owns and operates a fully permitted RCRA facility with the technical expertise Evoqua Water Technologies Wastewater Ion Exchange (WWIX) services and and equipment necessary to safely manage equipment help customers meet their wastewater handling challenges. Evoqua regeneration of the spent resins while provides system design, installation, and custom services that treat wastewater remaining environmentally compliant. contaminated with heavy metals. Zinc, copper, nickel and chromium are just a Where practical, the media/resin is recycled few examples of the metals that can be removed to low part per billion levels. Our for future use. We can accept both non- wastewater treatment systems are designed to meet your discharge requirements, hazardous and hazardous waste. achieve the water quality level needed for reuse and recycling and minimize the liability associated with on-site storage and handling of chemicals and wastes. Typical Applications Markets Every Application has Unique Requirements • Metal finishing, electroplating and Each application is examined to determine the system configuration that best coating meets current and future needs. The system components are selected based upon • Parts washing available manpower, space limitations, access limitation and the specific reuse or • Aerospace discharge quality required. If needs change, our wastewater treatment systems are • Anodizing flexible – we can simply change the media types and/or tank size saving our clients • Circuit board assembly and significant capital expense. manufacturing What is WWIX Service? • Microelectronics • EDM Ion exchange (IX) is a proven and cost-effective technology for removing inorganic • General manufacturing contaminants. Evoqua‘s WWIX service utilizes ion exchange resins and other • Groundwater remediation media selected to remove specific ionic contaminants from groundwater, industrial • Magnetic/digital media production wastewater, and process water for recycle. -

Wastewater Treatment by Electrodialysis System and Fouling Problems
The Online Journal of Science and Technology - January 2016 Volume 6, Issue 1 WASTEWATER TREATMENT BY ELECTRODIALYSIS SYSTEM AND FOULING PROBLEMS Elif OZTEKIN, Sureyya ALTIN Bulent Ecevit University, Department of Environmental Engineering, Zonguldak-Turkey [email protected], VDOWÕQ#NDUDHOPDVHGXWU Abstract: Electrodialysis ED is a separation process commercially used on a large scale for production of drinking water from water bodies and treatment of industrial effluents (Ruiz and et al., 2007). ED system contains ion exchange membranes and ions are transported through ion selective membranes from one solution to another under the influence of electrical potential difference used as a driving force. ED has been widely used in the desalination process and recovery of useful matters from effluents. The performance of ED, depends on the operating conditions and device structures such as ion content of raw water, current density, flow rate, membrane properties, feed concentration, geometry of cell compartments (Chang and et al., 2009, Mohammadi and et al., 2004). The efficiency of ED systems consist in a large part on the properties of the ion exchange membranes. Fouling of ion exchange membranes is one of the common problems in ED processes (Lee and et al., 2009, Ruiz and et al., 2007). Fouling is basically caused by the precipitation of foulants such as organics, colloids and biomass on the membrane surface or inside the membrane and fouling problem reduces the transport of ions. The fouling problems are occasion to increase membrane resistance, loss in selectivity of the membranes and affect negatively to membrane performance (Lee and et al., 2002, Lindstrand and et al., 2000a, Lindstrand and et al., 2000b). -
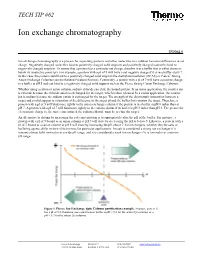
Ion Exchange Chromatography
TECH TIP #62 Ion exchange chromatography TR0062.0 Ion exchange chromatography is a process for separating proteins and other molecules in a solution based on differences in net charge. Negatively charged molecules bind to positively charged solid supports and positively charged molecules bind to negatively charged supports. To ensure that a protein has a particular net charge, dissolve it in a buffer that is either above or below its isoelectric point (pI). For example, a protein with a pI of 5 will have a net negative charge if it is in a buffer at pH 7. In this case, the protein could bind to a positively charged solid support like diethylaminoethanol (DEAE) or Pierce® Strong Anion Exchange Columns (see the Related Products Section). Conversely, a protein with a pI of 7 will have a positive charge in a buffer at pH 5 and can bind to a negatively charged solid support such as the Pierce Strong Cation Exchange Columns. Whether using a cation or anion column, sodium chloride can elute the bound protein. In an anion application, the counter ion is chloride because the chloride anion is exchanged for the target, which is then released. In a cation application, the counter ion is sodium because the sodium cation is exchanged for the target. The strength of the electrostatic interaction between a target and a solid support is a function of the difference in the target pI and the buffer that contains the target. Therefore, a protein with a pI of 5 will bind more tightly to the anion exchange column if the protein is in a buffer at pH 8 rather than at pH 7.