Status of Surface Water from Selected Areas of Coastal Guyana And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Attitudes Toward Homosexuals in Guyana (2013)
ATTITUDES TOWARD HOMOSEXUALS IN GUYANA (2013) Report prepared by CONTENTS SYNOPSIS ................................................................................................................................................ 4 ACKNOWLEDGEMENTS .......................................................................................................................... 6 INTRODUCTION, METHODOLOGY AND LIMITATIONS .......................................................................... 8 Table 01: ............................................................................................................................................ 8 Region of Interview .......................................................................................................................... 8 SURVEY DEMOGRAPHICS ..................................................................................................................... 11 Table 02: Sex of Respondent ....................................................................................................... 11 Table 03: Race of Respondent .................................................................................................... 11 Table 04: Age Range of Respondent .......................................................................................... 11 Table 05: Respondent’s Origin ..................................................................................................... 11 Table 06: Respondent’s Income Range .................................................................................... -

School Teachers in the Colony, 1860
Copyright 2007-8: School Teachers 1860 British Guiana Sharon Anderson in the Colony, 1860 Almanack Pg 121-123 Name of Teacher Church LAST FIRST School Locality Class Salary Alleyne John Church of Scotland All Saint's - Boys Unql. 133.33 Alsop James Church of England The Kitty II 480 Armstrong Sarah Church of England Christ Church II 320 Arthur S.E. Church of England The Craig II 400 Ashby Samuel Church of England Hackney (Pomeroon) Unql. 133.33 Bacon P.S. Church of England Plaisance, Boys I 720 Banks E.T. Church of England De Kinderen Unql. 133.33 Bean Quammy Church of England Goed Fortuin II 200 Benjamin S.W. Church of Scotland Ithaca II 400 Binning Ellen N. Church of Scotland Stanley Town Unql. 106.66 Blyden John Episcopalian Meth. Gladstoneville III 200 Bornall Elizabeth Church of England St. Philip's - Girls II 320 Brown John W. Wesleyan Mocha III 200 Callendar C.A. Church of England Louisiana III 200 Clyne A.N. Church of Scotland Vriesland III 200 Cooper Joseph B. Church of Scotland Uitvlugt I 720 Cubbison Edward A. Church of England St. George's - Boys I 720 Cumberbatch David Church of Scotland Beauvoisin III 200 Cuvilje Peter C. Wesleyan Mahaicony III 200 Dance Charles Church of England Beterverwagting II 480 Davis J.T. Wesleyan Victoria II 400 de Bakker George Wesleyan Virginia Unql. 133.33 Don Thomas Church of England Queen's Town II 400 Drew W.E. Wesleyan All Saints II 480 Eastman Emma Church of England St. George's - Girls II 320 Eburne Alex Church of England Enmore III 200 Emery Clementina Wesleyan Kingston, Girls II 320 Eversley B. -

April 2010 Worldwide Newsletter
ROTARY INTERNATIONAL—LITERACY RESOURCE GROUP World Wide Literacy Newsletter Volume 2, Issue 9 From the Desk of Roger Hayward—North American Area Coordinator April, 2010 Another Rotary year is beginning to wind down. This has been another amazing year Special points of for the work of literacy activities around the world. One of the keys that must happen is interest: continuity from one year to the next. The • Teacher Training second key is sustainability. Is what we are • Character Literacy doing sustainable? in North Carolina The Montreal convention will soon be upon Inside this issue: us. It is certainly not too late to travel to ROTARY READS!!! Canada, the relatively old city of Montreal, This is a catalogue of literacy projects, scored when the weather will be wonderful. The for ease of implementation, and is available convention promises to be very worthwhile on the Zone 24 website. Just access the home The Rotary Club 2 and the literacy booths will all be together of Bombay page, click on Literacy and Education on the again (bigger and better than last year!) and left hand side, then click on Rotary Reads, Santa Fe, New 3 two literacy breakout sessions are planned. again on the left hand side of the page. Mexico There will be more details in the May issue This is an excellent resource if your club is of this newsletter. looking for literacy programs. Let the 4 Children Speak The Rotary Club of Stabroek, Georgetown, Guyana Toilets and 4 The Rotary Club of Stabroek, Georgetown, math, English, science and social studies are taught Literacy Guyana, recently expanded its literacy project to and outdoor sessions where time is spent learning Region 5 ( Mahaica/ West Berbice), which is about sports such as volleyball, football and cricket. -

National Recount of Guyana's General and Regional Elections: Findings
National Recount of Guyana’s General and Regional Elections: Findings and Analysis of Observed Anomalies/ Irregularities Within Regions 1-10 2339 Ballot Boxes Examined EXECUTIVE SUMMARY Introduction The purpose of the enclosed reports (Region 1 to 10) is to present an analysis of the data captured from observed anomalies/irregularities during the national recount and determine its impact on the credibility of the 02 March 2020 General and Regional Elections (G&RE). The Party’s data analytical team interrogated observed anomalies/irregularities during the national recount under four main headings: Missing Poll Books; Poll Book Irregularities; Illegal Voting and Statistical Anomalies. The Missing Poll Books heading provided information on the number of missing Poll Books, the number of missing certificates of employment (COEs) and the number of unverified Oaths of Identity (OOIs) based on missing Poll Books. The Poll Book Irregularities heading provided information on missing entries in Poll Books, missing OOIs; mismatching of counterfoils; missing ballot box seals; missing Oaths of Blind/Incapacitated; missing unused, used and spoilt ballots; missing appointment letters and missing proxies. The Illegal Voting heading provided information on the number of deceased persons who voted, the number of migrated persons who voted while being out of Guyana at the time of the G&RE, and the number of persons who did not collect a national identification card (ID) but voted without any OOI. The Statistical Anomaly heading provided information on the instances of variance in the number of electors ticked by the various polling station staff as voted at a particular polling station. GB/ June 10/2020. -

Proceedings and Debates of The
PROCEEDINGS AND DEBATES OF THE NATIONAL ASSEMBLY OF THE FIRST SESSION (2020-2025) OF THE TWELFTH PARLIAMENT OF GUYANA UNDER THE CONSTITUTION OF THE CO-OPERATIVE REPUBLIC OF GUYANA HELD IN THE DOME OF THE ARTHUR CHUNG CONFERENCE CENTRE, LILIENDAAL, GREATER GEORGETOWN 6TH Sitting Thursday, 17TH September, 2020 The Assembly convened at 10.03 a.m. Prayers [Mr. Speaker in the Chair] MEMBERS OF THE NATIONAL ASSEMBLY (70) Speaker (1) *Hon. Manzoor Nadir, M.P., (Virtual Participation) Speaker of the National Assembly, Parliament Office, Public Buildings, Brickdam, Georgetown. MEMBERS OF THE GOVERNMENT (37) (i) MEMBERS OF THE PEOPLE’S PROGRESSIVE PARTY/CIVIC (PPP/C) (37) Prime Minister (1) + Hon. Brigadier (Ret’d) Mark Anthony Phillips, M.S.S., M.P., Prime Minister, Prime Minister’s Office, Colgrain House, 205 Camp Street, Georgetown. Vice-President (1) + Hon. Bharrat Jagdeo, M.P., Vice-President, Office of the President, New Garden Street, Georgetown. + Cabinet Member * Non-Elected Speaker Attorney General and Minister of Legal Affairs (1) + Hon. Mohabir Anil Nandlall, M.P., Attorney General and Minister of Legal Affairs, Ministry of Legal Affairs, Carmichael Street, Georgetown. Senior Ministers (16) + Hon. Gail Teixeira, M.P., (Region No. 7 – Cuyuni/Mazaruni), Minister of Parliamentary Affairs and Governance, Ministry of Parliamentary Affairs and Governance. Government Chief Whip, Office of the Presidency, New Garden Street, Georgetown. + Hon. Hugh H. Todd, M.P., [Absent - on Leave] (Region No. 4 – Demerara/Mahaica), Minister of Foreign Affairs and International Co-operation, Ministry of Foreign Affairs, Lot 254 South Road, Georgetown. + Hon. Bishop Juan A. Edghill, M.S., J.P., M.P., Minister of Public Works, Ministry of Public Works, Wight’s Lane, Kingston, Georgetown. -

Invitation for Bids Ciip
INVITATION FOR BIDS GOVERNMENT OF THE COOPERATIVE REPUBLIC OF GUYANA MINISTRY OF COMMUNITIES COMMUNITY INFRASTRUCTURE IMPROVEMENT PROJECT 1. The Ministry of Communities invites eligible bidders to submit their bids for the following works: 1. Construction of Sanitary Block at Hosororo Community Ground, Barima/Waini Region 2. Rehabilitation of Kumaka Marketing Centre, Mabaruma, Barima/Waini Region 3. Rehabilitation of Maria- Johanna Ground, Wakenaam, Essequibo Islands-West Demerara Region 4. Rehabilitation of Arthurville Ground, Wakenaam, Essequibo Islands-West Demerara Region 5. Construction of Community Centre Ground/ Facilities at Mocha/Arcadia NDC, Demerara- Mahaica 6. Upgrading of Paradise Playfield, Paradise Village, ECD, Demerara- Mahaica Region 7. Rehabilitation of Belladrum Multipurpose Centre, Mahaica-Berbice Region 8. Rehabilitation of Children’s Playgrounds in Blairmont/ Gelderland NDC, Mahaica- Berbice Region 9. Construction of Concrete Koker at Kortberaadt, East Berbice – Corentyne Region 10. Construction of Tabatinga Bridge Approach, Upper Takutu- Upper Essequibo Region 2. Interested bidders may obtain further information, familiarize and purchase a complete set of the bidding documents upon having a written request and after the payment of a non- refundable fee in the amount of Four Thousand Guyana dollars (4,000 GYD) from the Accounts Department, Ministry of Communities, Fort Street, Kingston, Georgetown. Tel: 592 226 5071-3, from February 28, 2017 during normal working hours. The method of payment will be cash. 3. All Bids must be accompanied by valid NIS and GRA Compliance Certificates. 4. All bidders should submit their bids together with the signed bid securing declaration form except for (2) above, where a bid security is required (the amount is specified in the Tender Document), no later than 09:00 hours on the “21st ” day of March, 2017 at the address below. -

Distr. Limited LC/CAR/L.88 31 October 2006 Original: English
______________________________________________________________________________ Distr. Limited LC/CAR/L.88 31 October 2006 Original: English GUYANA THE IMPACT ON SUSTAINABLE LIVELIHOODS CAUSED BY THE DECEMBER 2005 – FEBRUARY 2006 FLOODING In collaboration with the Inter-American Institute for Cooperation and Agriculture (IICA) ___________________ This document has been reproduced without formal editing i PREFACE This report was prepared at the request of the Government of Guyana following the rainfall from December 2005 until February 2006 and the subsequent flooding that affected, in particular, Regions 2 and 5. The implications of the flooding posed the need, beyond the immediate humanitarian response, for a rapid assessment of the damages (impacts of assets) and losses (effects on economic and social flows) to determine at the more general level the implications on macroeconomic and fiscal performance and the social and environmental consequences.1 More specifically, the document examines the effects of the impact for the sustainable livelihood of the affected households and provides possible strategic interventions serving as inputs for the development of programmes and projects to mitigate the impact of flooding on the affected population. The assessment was carried out following the Economic Commission for Latin America and the Caribbean (ECLAC) disaster assessment methodology as elaborated in the Handbook for Estimating the Socio-economic and Environmental Effects of Disasters (ECLAC, 2004, www.eclac.cl/mexico). The results of the assessment were also interpreted within the context of a sustainable livelihood analysis (SLA) framework. The SLA framework combines the concept of sustainability understood as comprising of four elements (environmental, economic, social and institutional) with that of livelihoods which in turn refer to the capabilities, assets and activities required for a means of living. -

Report of the Auditor General for the Fiscal Year
31 October 2002 Hon. Ralph Ramkarran, S.C., M.P., Speaker of the National Assembly Public Buildings, Avenue of the Republic, Georgetown. Dear Mr. Speaker, REPORT OF THE AUDITOR GENERAL ON THE PUBLIC ACCOUNTS OF GUYANA AND ON THE ACCOUNTS OF MINISTRIES, DEPARTMENTS AND REGIONS FOR THE FISCAL YEAR ENDED 31 DECEMBER 2001 In accordance with Article 223(2) of the Constitution of the Republic of Guyana, I am pleased to submit the attached report on the Public Accounts of Guyana and on the Accounts of the Ministries/Departments/Regions for the fiscal year ended 31 December 2001. The report is required to be laid before the National Assembly, and I would very much appreciate if this is done at the earliest opportunity. With kind regards. Yours sincerely, B. BALRAM AUDITOR GENERAL(Ag.) REPORT OF THE AUDITOR GENERAL ON THE PUBLIC ACCOUNTS OF GUYANA AND ON THE ACCOUNTS OF THE MINISTRIES, DEPARTMENTS AND REGIONS FOR THE FISCAL YEAR ENDED 31 DECEMBER 2001 TABLE OF CONTENTS PAGE Audit Certificate i Highlights of the Report iii Introduction 1 Report on the Public Accounts Statements: Receipts and Payments of the Consolidated Fund 4 Revenue Actually Paid into the Consolidated Fund as Compared with the Estimates of Revenue 12 Expenditure from the Consolidated Fund as Compared with the Estimates of Expenditure 14 The Public Debt 15 Outstanding Loans or Credits Guaranteed by the Government 17 Outstanding Loans and Advances made from the Consolidated Fund 18 Statutory Expenditure 20 Receipts and Payments of the Contingencies Fund 22 Balances held on Deposit -

Region 5 Gets New Rehab Center Georgetown, GINA, June 22, 2010
Region 5 gets new rehab center Georgetown, GINA, June 22, 2010 A new rehabilitation center is to be commissioned in Wednesday at the Mahaicony Diagnostic Center. The clinic there is expected to cater to the thousands of residents in Region 5 and its environs. Chairman of Region 5 (Mahaica/ Berbice) Harrinarine Baldeo said the establishment of the center stands testimony of government’s commitment to the wellbeing and health of its citizens especially those of his region. The chairman said that the health sector in Region 5 has seen significant improvements over the years. According to recent statistics, there are now two district hospitals which function optimally, 17 health centers, and one health post at No. 28 Village. The newly built Rehab Center at the Mahaicony Diagnostic Center In addition to the new rehab center, the Mahaicony Diagnostic Center, offers clinics in chronic diseases and eye care. There are 24 medical personnel, among them Cuban doctors, stationed there as part of the Guyana/Cuba collaboration initiative by President Jagdeo in 2006. At Fort Wellington, the full service hospital there is staffed by four local doctors, assisted by nurses, who are trained to deal with from seizures to surgeries. The more serious cases are referred to Mahaicony or Georgetown. Before 1992, Mr. Baldeo said, this was hardly a possibility. Rosignol Health Center Throughout the region, there are four medex that staff health centers at Belladrum, Ithaca, Moraikobai and Woodley Park. There are also community health workers stationed at other clinics there. This, some residents say was not a situation that existed prior to 1992. -

28 April, 2000 Hon. Sasenarine Kowlessar, Minister Responsible For
28 April, 2000 Hon. Sasenarine Kowlessar, Minister Responsible for Finance, Ministry of Finance, Main & Urquhart Streets, Georgetown. Dear Minister, REPORT OF THE AUDITOR GENERAL ON THE PUBLIC ACCOUNTS OF GUYANA AND ON THE ACCOUNTS OF MINISTRIES, DEPARTMENTS AND REGIONS FOR THE FISCAL YEAR ENDED 31 DECEMBER 1998 In accordance with Article 223(2) of the Constitution of the Republic of Guyana, I am pleased to submit the attached report on the Public Accounts of Guyana and on the Accounts of the Ministries/Departments/Regions for the fiscal year ended 31 December 1998. The report is required to be laid before the National Assembly, and I would very much appreciate if this is done at the earliest opportunity. With kind regards. Yours sincerely, ANAND GOOLSARRAN AUDITOR GENERAL REPORT OF THE AUDITOR GENERAL ON THE PUBLIC ACCOUNTS OF GUYANA AND ON THE ACCOUNTS OF THE MINISTRIES, DEPARTMENTS AND REGIONS FOR THE FISCAL YEAR ENDED 31 DECEMBER 1998 TABLE OF CONTENTS PAGE Audit Certificate i Highlights of the Report iv Introduction 1 Report on the Public Accounts Statements: Receipts and Payments of the Consolidated Fund 5 Revenue Actually Paid into the Consolidated Fund as Compared with the Estimates of Revenue 13 Expenditure from the Consolidated Fund as Compared with the Estimates of Expenditure 15 The Public Debt 17 Outstanding Loans or Credits Guaranteed by the Government 19 Outstanding Loans and Advances made from the Consolidated Fund 20 Statutory Expenditure 22 Receipts and Payments of the Contingencies Fund 23 Balances held on Deposit by -

Guyana (Country Code +592) Communication of 9.II.2015: Guyana Telephone and Telegraph Co
Guyana (country code +592) Communication of 9.II.2015: Guyana Telephone and Telegraph Co. Ltd, Georgetown, announces an update to the national numbering plan of Guyana with cellular code blocks for Digicel Guyana lncorporated, a registered cellular operator in Guyana. NDC - National N(S)N Number Length Destination Code or Usage of E.164 Additional leading digits of N(S)N – Maximum Minimum number Information National Significant Length Length Number 600XXXX to 604XXXX 7 7 Non-geographic Digicel Guyana Inc. number – Mobile telephony service 659XXXX 7 7 Non-geographic Digicel Guyana Inc. number – Mobile telephony service 660XXXX to 669XXXX 7 7 Non-geographic Digicel Guyana Inc. number – Mobile telephony service 670XXXX to 674XXXX 7 7 Non-geographic Digicel Guyana Inc. number – Mobile telephony service 675XXXX to 679XXXX 7 7 Non-geographic Digicel Guyana Inc. number – Mobile telephony service 680XXXX to 689XXXX 7 7 Non-geographic Digicel Guyana Inc. number – Mobile telephony service 690XXXX to 699XXXX 7 7 Non-geographic Digicel Guyana Inc. number – Mobile telephony service Contact: Mr Clinton Francis Supervisor – Business Planning Guyana Telephone & Telegraph Co. Ltd. 79 Brickdam, Stabroek P.O. Box 10628 GEORGETOWN Guyana Tel: +592 223 8024 Fax: +592 226 8940 E-mail: [email protected] Communication of 31.XII.2009: Guyana Telephone & Telegraph Company Limited (GT&T), Georgetown, announces the introduction of the following new area codes and number ranges: Location Area codes and number ranges Mocha E.B.D. 217 0XXX, 217 1XXX (new wire-line switch) Crabwood Creek, Corentyne 335 1XXX (upgrade) Hope West, E.C.D. 256 5XXX (upgrade) Enterprise E.C.D. -
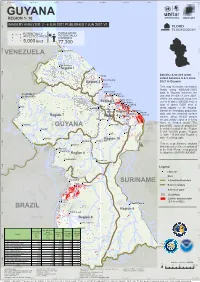
Guyana 9°45'N Region 1- 10 Imagery Analysis: 2 - 6 Jun 2021 Published 7 Jun 2021 V1
61°30'W 60°45'W 60°W 59°15'W 58°30'W 57°45'W 57°W 56°15'W 55°30'W 9°45'N GUYANA 9°45'N REGION 1- 10 IMAGERY ANALYSIS: 2 - 6 JUN 2021 PUBLISHED 7 JUN 2021 V1. FLOOD 9°N FL20210520GUY 9°N POTENTIALLY POPULATION AFFECTED AREA POTENTIALLY EXPOSED 5,000 km2 77,300 Mabaruma 8°15'N VENEZUELA 8°15'N Port Kaituma Region 1 7°30'N Satellite detected water Charity 7°30'N extent between 2 & 6 June Anna Regina Region 2 2021 in Guyana Huist T'Dieren This map illustrates cumulative floods using NOAA20-VIIRS Vergenogen data in Guyana between the San Martín Georgetown de Turumbang Plaisance Buxton 2nd and the 6th of June 2021. 6°45'N Cane Grove Within the analyzed cloud free 6°45'N Region 3 zones of about 200,000 km2, a Timehri Little Biabu Perth Lichfield total of about 5,000 km2 of Arau Bartica St. Belladrum lands appear to be flooded. Agatash Cuthbert's Rosignol Based on Worldpop population Mission Region 5 Alness Belvedere data and the detected surface Region 7 Bloomfield Region 4 Morikaibai waters, about 80,000 people Rockstone Linden are potentially exposed or living 6°N close to flooded areas. The 6°N Paruima GUYANA Lancaster potentially exposed population Jawalla is mainly located in the Region Ebini 6 with ~22,000 people, Region Ituni 3 with ~19,000 and Region 5 Pipillipai Region 10 with ~15,000 people. Mahdia Mabura Hill This is a preliminary analysis 5°15'N Kaieteur that has not yet been validated 5°15'N Kaibarupai in the field.