IMI-NFG Course on Processing in Glass Lecture 1: Commercial Glass
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aremco—High Temperature Solutions
High Temperature Solutions Since 1965, our success has been a result of this simple business strategy: • Understanding Customer Requirements. • Providing Outstanding Service and Support. • Producing High Quality Technical Materials and Equipment. • Solving Difficult Technical Problems. CONTENTS Technical Bulletin Page No. A1 Machinable & Dense Ceramics .......................................................... 2 A2 High Temperature Ceramic & Graphite Adhesives ....................... 6 A3 High Temperature Ceramic-Metallic Pastes .................................12 A4 High Temperature Potting & Casting Materials ...........................14 A5-S1 High Temperature Electrical Coatings & Sealants ......................18 A5-S2 High Temperature High Emissivity Coatings ................................20 A5-S3 High Temperature Thermal Spray Sealants ..................................22 A5-S4 High Temperature Coatings for Ceramics, Glass & Quartz ......24 A5-S5 High Temperature Refractory Coatings .........................................26 A6 High Temperature Protective Coatings ..........................................28 A7 High Performance Epoxies ................................................................34 A8 Electrically & Thermally Conductive Materials .............................36 A9 Mounting Adhesives & Accessories ................................................38 A10 High Temperature Tapes ...................................................................42 A11 High Temperature Inorganic Binders..............................................44 -

Grand Canyon.Com's Spring Travel Guide
Grand Canyon.com’s Spring Travel Guide Second Edition Helping You Get Even More Out of Your Grand Canyon Vacation! Thank you for choosing Grand Canyon.com as your Southwest destination specialist! You’ve chosen a truly extraordinary place for your spring vacation, and our mission is to help you get the most out of your trip. Having helped thousands of busy people like you plan their Grand Canyon vacations for over 20 years, our staff has made a few observations and picked up a few insider tips that can help save you time, money and hassle - sometimes all three at once! It was to that end that we presented our First Annual Spring Break Travel Guide in February. Since then, peoples’ response has been nothing short of overwhelming. But with spring break extending well into April this year, we realized that a few things needed updating in order for you to be as well informed as possible before hitting the road. It is to that end that we present: Grand Canyon.com’s First Annual Spring Travel Guide: The Second Edition Before you dig in, we recommend that you grab a few things: a map or road atlas, a pen and/or a highlighter, maybe a beverage, a few minutes of quiet time, and your “Grand Canyon Top Tours Brochure.” Let’s get started and get YOU* to the Grand Canyon! *Got most of your trip figured out already? Skip to Chapter 8 Traveler Tip 1 - Where’s It At and What Side Am I On? The Grand Canyon is in Northern Arizona. -

Fiber Optic Cable for VOICE and DATA TRANSMISSION Delivering Solutions Fiber Optic THAT KEEP YOU CONNECTED Cable Products QUALITY
Fiber Optic Cable FOR VOICE AND DATA TRANSMISSION Delivering Solutions Fiber Optic THAT KEEP YOU CONNECTED Cable Products QUALITY General Cable is committed to developing, producing, This catalog contains in-depth and marketing products that exceed performance, information on the General Cable quality, value and safety requirements of our line of fiber optic cable for voice, customers. General Cable’s goal and objectives video and data transmission. reflect this commitment, whether it’s through our focus on customer service, continuous improvement The product and technical and manufacturing excellence demonstrated by our sections feature the latest TL9000-registered business management system, information on fiber optic cable the independent third-party certification of our products, from applications and products, or the development of new and innovative construction to detailed technical products. Our aim is to deliver superior performance from all of General Cable’s processes and to strive for and specific data. world-class quality throughout our operations. Our products are readily available through our network of authorized stocking distributors and distribution centers. ® We are dedicated to customer TIA 568 C.3 service and satisfaction – so call our team of professionally trained sales personnel to meet your application needs. Fiber Optic Cable for the 21st Century CUSTOMER SERVICE All information in this catalog is presented solely as a guide to product selection and is believed to be reliable. All printing errors are subject to General Cable is dedicated to customer service correction in subsequent releases of this catalog. and satisfaction. Call our team of professionally Although General Cable has taken precautions to ensure the accuracy of the product specifications trained sales associates at at the time of publication, the specifications of all products contained herein are subject to change without notice. -

Fluidized Bed Chemical Vapor Deposition of Zirconium Nitride Films
INL/JOU-17-42260-Revision-0 Fluidized Bed Chemical Vapor Deposition of Zirconium Nitride Films Dennis D. Keiser, Jr, Delia Perez-Nunez, Sean M. McDeavitt, Marie Y. Arrieta July 2017 The INL is a U.S. Department of Energy National Laboratory operated by Battelle Energy Alliance INL/JOU-17-42260-Revision-0 Fluidized Bed Chemical Vapor Deposition of Zirconium Nitride Films Dennis D. Keiser, Jr, Delia Perez-Nunez, Sean M. McDeavitt, Marie Y. Arrieta July 2017 Idaho National Laboratory Idaho Falls, Idaho 83415 http://www.inl.gov Prepared for the U.S. Department of Energy Under DOE Idaho Operations Office Contract DE-AC07-05ID14517 Fluidized Bed Chemical Vapor Deposition of Zirconium Nitride Films a b c c Marie Y. Arrieta, Dennis D. Keiser Jr., Delia Perez-Nunez, * and Sean M. McDeavitt a Sandia National Laboratories, Albuquerque, New Mexico 87185 b Idaho National Laboratory, Idaho Falls, Idaho 83401 c Texas A&M University, Department of Nuclear Engineering, College Station, Texas 77840 Received November 11, 2016 Accepted for Publication May 23, 2017 Abstract — – A fluidized bed chemical vapor deposition (FB-CVD) process was designed and established in a two-part experiment to produce zirconium nitride barrier coatings on uranium-molybdenum particles for a reduced enrichment dispersion fuel concept. A hot-wall, inverted fluidized bed reaction vessel was developed for this process, and coatings were produced from thermal decomposition of the metallo-organic precursor tetrakis(dimethylamino)zirconium (TDMAZ) in high- purity argon gas. Experiments were executed at atmospheric pressure and low substrate temperatures (i.e., 500 to 550 K). Deposited coatings were characterized using scanning electron microscopy, energy dispersive spectroscopy, and wavelength dis-persive spectroscopy. -

Acrylic Polymer Transparencies
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 4-1972 Acrylic Polymer Transparencies Inez Allen Kendrick Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Art Education Commons, Art Practice Commons, and the Interdisciplinary Arts and Media Commons Let us know how access to this document benefits ou.y Recommended Citation Kendrick, Inez Allen, "Acrylic Polymer Transparencies" (1972). Dissertations and Theses. Paper 1554. https://doi.org/10.15760/etd.1553 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. AN ABSTRACT OF T.HE THESIS OF Inez Allen Kendrick tor the Master ot Science in Teaching in Art presented April 26, 1972. TITLE: Acrylic Polymer Transparencies. APPROVED BY MEMBERS 01' 'lBE THESIS COMMITTEE: Richard J. ~asch, Chairman ~d B. Kimbrell Robert S. Morton Brief mentions by three writers on synthetic painting media first intrigued my interest in a' new technique of making transparent acrylic paintings on glass or plexiglas supports, some ot which were said to I I simulate stained-glass windows. In writing this paper on acrylic polymer'", transparencies, 'my problem was three-told: first. to determine whether any major recognized works of art have been produced by this, method; second, to experiment with the techni'que and materials in order to explore their possibilities for my own work; and third, to determine whether both materials and methods would be suitable for use in a classroom. -

Business Grand Canyon SKYWALK Preview Betting to 'Improve Our Lot,' Hualapai Tribe Puts up $30M Opens to Public Next Week; Be Prepared to Pay by Levi J
Business Grand Canyon SKYWALK Preview Betting to 'improve our lot,' Hualapai Tribe puts up $30M Opens to public next week; be prepared to pay By Levi J. Long ARIZONA DAILY STAR Tucson, Arizona | Published: 03.21.2007 The Colorado River flows more than 4,000 feet below the Hualapai Tribe's Skywalk, which swings 70 feet out from the edge of Grand Canyon West, near Kingman. DEAN KNUTH / arizona daily star GRAND CANYON WEST — The Hualapai Tribe is betting $30 million that an unpaved transportation artery leading to a remote stretch of the Grand Canyon rim will pump life into the tribe's tourism-based economy. On Tuesday, Hualapai officials invited hundreds of VIPs and members of the news media to tour the tribe's new steel and glass-bottomed walkway, jutting 70 feet past the canyon's dusty edge. Their hope: It will funnel thousands more tourists, and their dollars, to the remote reservation. The Skywalk, which is scheduled to open to the public March 28, soars about 4,000 feet above the canyon floor. Visitors who are not faint of heart and willing to pay $75 each can get a bird's eye view of the canyon from the horseshoe-shaped walkway. Under construction since mid-2005, the Skywalk has received worldwide attention, thrusting the 2,300-member Hualapai Tribe into a media whirlwind. "This is the only one of its kind in the world, and it's on our reservation," said Waylon Honga, chief operating officer for Grand Canyon Resort Corp., which guides reservation business and tourism development. -
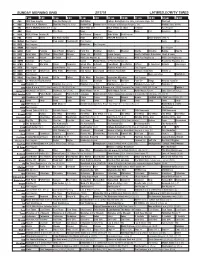
Sunday Morning Grid 2/17/19 Latimes.Com/Tv Times
SUNDAY MORNING GRID 2/17/19 LATIMES.COM/TV TIMES 7 am 7:30 8 am 8:30 9 am 9:30 10 am 10:30 11 am 11:30 12 pm 12:30 2 CBS CBS News Sunday Face the Nation (N) Bull Riding College Basketball Ohio State at Michigan State. (N) PGA Golf 4 NBC Today in L.A. Weekend Meet the Press (N) (TVG) Hockey Day Hockey New York Rangers at Pittsburgh Penguins. (N) Hockey: Blues at Wild 5 CW KTLA 5 Morning News at 7 (N) Å KTLA News at 9 KTLA 5 News at 10am In Touch Paid Program 7 ABC News This Week News News News Paid American Paid 9 KCAL KCAL 9 News Sunday (N) Joel Osteen Jentzen Mike Webb Paid Program 1 1 FOX Planet Weird Fox News Sunday News PBC Face NASCAR RaceDay (N) 2019 Daytona 500 (N) 1 3 MyNet Paid Program Fred Jordan Freethought Paid Program News Paid 1 8 KSCI Paid Program Buddhism Paid Program 2 2 KWHY Paid Program Paid Program 2 4 KVCR Paint Painting Joy of Paint Wyland’s Paint This Painting Kitchen Mexican Martha Christina Baking How To 2 8 KCET Zula Patrol Zula Patrol Mixed Nutz Edisons Curios -ity Biz Kid$ Grand Canyon Huell’s California Adventures: Huell & Louie 3 0 ION Jeremiah Youseff In Touch Paid NCIS: Los Angeles Å NCIS: Los Angeles Å NCIS: Los Angeles Å NCIS: Los Angeles Å 3 4 KMEX Conexión Paid Program Fútbol Fútbol Mexicano Primera División (N) República Deportiva (N) 4 0 KTBN Jeffress Win Walk Prince Carpenter Intend Min. -

Thermal Runaway Severity Reduction Assessment for EVA Li-Ion Batteries
https://ntrs.nasa.gov/search.jsp?R=20150018568 2019-08-31T06:01:42+00:00Z Thermal Runaway Severity Reduction Assessment For EVA Li-ion Batteries By Eric Darcy/NASA-JSC For 2014 JSC Connect Event Energy Storage & Management 24 Sept 2014 Team and Contents 2 • NASA Engineering Safety Center Lead Effort – Paul Shack, Assessment Lead – Chris Iannello, NESC Technical Fellow for Electrical Power – Steve Rickman, NESC Technical Fellow for Passive Thermal – Eric Darcy, Test Lead for EVA Batteries, NASA-JSC – Sam Russell, Mike Fowler, Judy Jeevarajan, Craig Clark, John Weintritt, Christina Deoja and Stacie Cox/NASA-JSC – Rob Button, Tom Miller, Penni Dalton/NASA-GRC – Dan Doughty, Bruce Drolen, Ralph White, Gary Bayles, and Jim Womack/NESC Consultants • Agenda – Background on the EVA batteries – Motivation and objectives – Trigger method selected and why – Assessments of current designs – Verification of subscale mitigation measures – Full scale LREBA with those measures leads to failure – Consequence of cell TR ejecta products to TR propagation – Full scale LREBA with adjacent cells protected from cell vent path – Bank test to verify benefits of cell fusing – Lessons learned to date Background - Li-ion Rechargeable EVA Battery Assembly (LREBA) 1 9P-5S Array of Samsung 2.6Ah 18650 cells to power the spacesuit helmet lights and camera and glove heaters Background – Li-ion Pistol Grip Tool Battery • 10-cell Li-ion 18650 battery – 10S for discharge – 2P-5S for charge • Battery is enclosed in tool holster except for end with the D-latch Background -
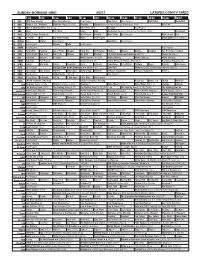
Sunday Morning Grid 4/2/17 Latimes.Com/Tv Times
SUNDAY MORNING GRID 4/2/17 LATIMES.COM/TV TIMES 7 am 7:30 8 am 8:30 9 am 9:30 10 am 10:30 11 am 11:30 12 pm 12:30 2 CBS CBS News Sunday Face the Nation (N) Paid Program Raw Travel Paid Program Bull Riding Basketball 4 NBC Today in L.A.: Weekend Meet the Press (N) (TVG) Pregame Hockey Boston Bruins at Chicago Blackhawks. (N) Å PGA Golf 5 CW KTLA 5 Morning News at 7 (N) Å KTLA News at 9 In Touch Paid Program 7 ABC News This Week News NBA Basketball Boston Celtics at New York Knicks. (N) Å Basketball 9 KCAL KCAL 9 News Sunday (N) Joel Osteen Schuller Mike Webb Paid Program REAL-Diego Paid 11 FOX In Touch Paid Fox News Sunday News Paid Program Dead Again ››› (1991) 13 MyNet Paid Matter Paid Program Best Buys Paid Program 18 KSCI Paid Program Church Faith Paid Program 22 KWHY Paid Program Paid Program 24 KVCR Paint With Painting Joy of Paint Wyland’s Paint This Oil Painting Kitchen Mexico Martha Cooking Fun N’ Simple Cooking 28 KCET 1001 Nights Bali (TVG) Bali (TVG) Edisons Biz Kid$ Biz Kid$ Ed Slott’s Retirement Roadmap 2017 Å Vibrant for Life Å 30 ION Jeremiah Youssef In Touch White Collar In the Wind. White Collar Å White Collar Å White Collar Å 34 KMEX Conexión Paid Program Fútbol Central (N) Fútbol Mexicano Primera División (N) República Deportiva (N) 40 KTBN James Win Walk Prince Carpenter Jesse In Touch PowerPoint It Is Written Pathway Super Kelinda John Hagee 46 KFTR Paid Program Ice Age: Dawn of the Dinosaurs ›› (2009) (PG) Zona NBA Killers › (2010, Acción) Ashton Kutcher. -

Stability of Materials for Use in Space-Based Interferometric Missions
STABILITY OF MATERIALS FOR USE IN SPACE-BASED INTERFEROMETRIC MISSIONS By ALIX PRESTON A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2010 1 °c 2010 Alix Preston 2 This is dedicated to all who were told they would fail, only to prove them wrong 3 ACKNOWLEDGMENTS Much of this work would not have been made possible if it were not for the help of many graduate and undergraduate students, faculty, and sta®. I would like to thank Ira Thorpe, Rachel Cruz, Vinzenz Vand, and Josep Sanjuan for their help and thoughtful discussions that were instrumental in understanding the nuances of my research. I would also like to thank Gabriel Boothe, Aaron Spector, Benjamin Balaban, Darsa Donelon, Kendall Ackley, and Scott Rager for their dedication and persistence to getting the job done. A special thanks is due for the physics machine shop, especially Marc Link and Bill Malphurs, who spent many hours on the countless projects I needed. Lastly, I would like to thank my advisor, Dr. Guido Mueller, who put up with me, guided me, and supported me in my research. 4 TABLE OF CONTENTS page ACKNOWLEDGMENTS ................................. 4 LIST OF TABLES ..................................... 9 LIST OF FIGURES .................................... 10 KEY TO ABBREVIATIONS ............................... 17 KEY TO SYMBOLS .................................... 19 ABSTRACT ........................................ 20 CHAPTER 1 INTRODUCTION .................................. 22 1.1 Space-Based Missions .............................. 23 1.2 GRACE ..................................... 23 1.3 GRACE Follow-On ............................... 25 1.4 LISA ....................................... 26 1.4.1 Introduction ............................... 26 1.4.2 Sources .................................. 27 1.4.2.1 Cosmological background sources ............. -

ARIZONA TRAVEL GUIDE Antelope Canyon
UK/ENGLISH DISCOVER UNFORGETTABLE PLACES & AMAZING WONDERS TRAVEL GUIDE 2020 ARIZONA STATE MAP St. George U T A H GLEN CANYON Colorado KAIBAB- FOUR Littlefield 15 PAIUTE Fredonia MONUMENT City VERMILION Page VALLEY CORNERS 389 CLIFFS Lees Ferry 160 TRIBAL ? 163 TRIBAL PARK Mexican Teec PARK PIPE Jacob Marble Water Nos SPRING Lake Canyon Pos ALT Kayenta 89 NAVAJO Vermilion 20 NEVADA Cliffs 98 Shonto 59 191 Round r e Rock v i Cow R 67 Springs GRAND CANYON— 89 SAN JUAN 12 GRAND SOUTHERN PARASHANT o NAVAJO Many CANYON d PAIUTE a Tsaile r Farms o Las Vegas er GRAND l Tonalea iv Supai o CANYON C Tuba R HUALAPAI City 160 NORTHERN 64 North Rim Temple HILLTOP HAVASUPAI Chinle CANYON HOOVER DE CHELLY Bar Grand DAM SKYWALK Moenkopi o Canyon LAKE Meadview GRAND d MEAD a Village ? CANYON r 264 o Tusayan Hotevilla l 18 64 Walpi o Polacca C Cameron Keams Fort 1 Oraibi Canyon 64 Gray Kykotsmovi Defiance HUALAPAI Mountain Second 93 Mesa Ganado 264 Peach ? Window Springs Valle 89 HOPI HUBBELL TRADING St. Michaels Rock 66 WUPATKI 6 POST Chloride 180 2 87 Indian 15 12 ? SUNSET Wells Valentine 64 CRATER 191 Bullhead Seligman Ash Leupp Lupton Laughlin City 68 Fork ? ? Flagstaff 15 ? ? ? 40 Chambers Williams RIORDAN 77 Sanders Kingman MANSION WALNUT 95 CANYON HOMOLOVI Oatman 89 DEAD HORSE ? Joseph Navajo 191 FORT RANCH ALT MOJAVE 89 Winslow City PETRIFIED HUALAPAI SLIDE ROCK 40 FOREST 61 Paulden TUZIGOOT Needles Sedona ? Mormon Lake Topock Chino Clarkdale RED Holbrook ? WEST JEROME Cottonwood ROCK Valley ? 87 Wikieup Jerome Village ? 179 of ALT Oak Creek 61 95 COAST Prescott Valley 89 260 377 180 ZUNI Bagdad MONTEZUMA CASTLE 77 ? Lake Montezuma ? Lake Havasu 169 FORT VERDE 93 Prescott YAVAPAI- ? ? City ? PRESCOTT Dewey ?Camp 260 St. -

Chemical Vapor Deposition of Silanes and Patterning on Silicon
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2010-12-15 Chemical Vapor Deposition of Silanes and Patterning on Silicon Feng Zhang Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Biochemistry Commons, and the Chemistry Commons BYU ScholarsArchive Citation Zhang, Feng, "Chemical Vapor Deposition of Silanes and Patterning on Silicon" (2010). Theses and Dissertations. 2902. https://scholarsarchive.byu.edu/etd/2902 This Dissertation is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Chemical Vapor Deposition of Silanes and Patterning on Silicon Feng Zhang A dissertation submitted to the faculty of Brigham Young University In partial fulfillment of the requirements for the degree of Doctor of Philosophy Matthew R. Linford Milton L. Lee Matthew C. Asplund Daniel E. Austin Robert C. Davis Department of Chemistry and Biochemistry Brigham Young University April 2011 Copyright © 2011 Feng Zhang All Rights Reserved ABSTRACT Chemical Vapor Deposition of Silanes and Patterning on Silicon Feng Zhang Department of Chemistry and Biochemistry Doctor of Philosophy Self assembled monolayers (SAMs) are widely used for surface modification. Alkylsilane monolayers are one of the most widely deposited and studied SAMs. My work focuses on the preparation, patterning, and application of alkysilane monolayers. 3-aminopropyltriethoxysilane (APTES) is one of the most popular silanes used to make active surfaces for surface modification. To possibly improve the surface physical properties and increase options for processing this material, I prepared and studied a series of amino silane surfaces on silicon/silicon dioxide from APTES and two other related silanes by chemical vapor deposition (CVD).