This Is the Published Version of a Paper Published in Journal of Experimental Botany. Citation for Th
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -
Estimating Numbers of Embryonic Lethals in Conifers
Heredity 69(1992)308—314 Received 26 November 1991 OThe Genetical Society of Great Britain Estimating numbers of embryonic lethals in conifers OUTI SAVOLAINEN, KATRI KARKKAINEN & HELMI KUITTINEN* Department of Genetics, University of Oulu, Oulu, Fin/and and *Department of Genetics, University of Helsinki, He/sink,, Fin/and Conifershave recessive lethal genes that eliminate most selfed embryos during seed development. It has been estimated that Scots pine has, on average, nine recessive lethals which act during seed development. Such high numbers are not consistent with the level of outcrossing, about 0.9—0.95, which has been observed in natural populations. Correcting for environmental mortality or using partial selfings provides significantly lower estimates of lethals. A similar discordance with numbers of lethals and observed outcrossing rates is true for other species. Keywords:embryoniclethals, inbreeding depression, outcrossing, Pinus sylvestris, Picea omorika. Introduction Reproduction system of conifers Conifershave no self-incompatibility mechanisms but Theproportion of self-pollination in conifers is early-acting inbreeding depression eliminates selfed variable. Sarvas (1962) suggested an average of 26 per embryos before seed maturation (Sarvas, 1962; cent for Pinus sylvestris, while Koski (1970) estimated Hagman & Mikkola, 1963). A genetic model for this values of self-fertilization around 10 per cent. The inbreeding depression has been developed by Koski genera Pinus and Picea have polyzygotic poly- (1971) and Bramlett & Popham (1971). Koski (1971, embryony, i.e. the ovules contain several archegonia. In 1973) has estimated that Pinus sylvestris and Picea Pinus sylvestris, the most common number of arche- abies have on average nine and 10 recessive lethals, gonia is two but it can range from one to five. -

Early Photosynthetic Eukaryotes Inhabited Low-Salinity Habitats
Early photosynthetic eukaryotes inhabited PNAS PLUS low-salinity habitats Patricia Sánchez-Baracaldoa,1, John A. Ravenb,c, Davide Pisanid,e, and Andrew H. Knollf aSchool of Geographical Sciences, University of Bristol, Bristol BS8 1SS, United Kingdom; bDivision of Plant Science, University of Dundee at the James Hutton Institute, Dundee DD2 5DA, United Kingdom; cPlant Functional Biology and Climate Change Cluster, University of Technology Sydney, Ultimo, NSW 2007, Australia; dSchool of Biological Sciences, University of Bristol, Bristol BS8 1TH, United Kingdom; eSchool of Earth Sciences, University of Bristol, Bristol BS8 1TH, United Kingdom; and fDepartment of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138 Edited by Peter R. Crane, Oak Spring Garden Foundation, Upperville, Virginia, and approved July 7, 2017 (received for review December 7, 2016) The early evolutionary history of the chloroplast lineage remains estimates for the origin of plastids ranging over 800 My (7). At the an open question. It is widely accepted that the endosymbiosis that same time, the ecological setting in which this endosymbiotic event established the chloroplast lineage in eukaryotes can be traced occurred has not been fully explored (8), partly because of phy- back to a single event, in which a cyanobacterium was incorpo- logenetic uncertainties and preservational biases of the fossil re- rated into a protistan host. It is still unclear, however, which cord. Phylogenomics and trait evolution analysis have pointed to a Cyanobacteria are most closely related to the chloroplast, when the freshwater origin for Cyanobacteria (9–11), providing an approach plastid lineage first evolved, and in what habitats this endosym- to address the early diversification of terrestrial biota for which the biotic event occurred. -

Algae & Marine Plants of Point Reyes
Algae & Marine Plants of Point Reyes Green Algae or Chlorophyta Genus/Species Common Name Acrosiphonia coalita Green rope, Tangled weed Blidingia minima Blidingia minima var. vexata Dwarf sea hair Bryopsis corticulans Cladophora columbiana Green tuft alga Codium fragile subsp. californicum Sea staghorn Codium setchellii Smooth spongy cushion, Green spongy cushion Trentepohlia aurea Ulva californica Ulva fenestrata Sea lettuce Ulva intestinalis Sea hair, Sea lettuce, Gutweed, Grass kelp Ulva linza Ulva taeniata Urospora sp. Brown Algae or Ochrophyta Genus/Species Common Name Alaria marginata Ribbon kelp, Winged kelp Analipus japonicus Fir branch seaweed, Sea fir Coilodesme californica Dactylosiphon bullosus Desmarestia herbacea Desmarestia latifrons Egregia menziesii Feather boa Fucus distichus Bladderwrack, Rockweed Haplogloia andersonii Anderson's gooey brown Laminaria setchellii Southern stiff-stiped kelp Laminaria sinclairii Leathesia marina Sea cauliflower Melanosiphon intestinalis Twisted sea tubes Nereocystis luetkeana Bull kelp, Bullwhip kelp, Bladder wrack, Edible kelp, Ribbon kelp Pelvetiopsis limitata Petalonia fascia False kelp Petrospongium rugosum Phaeostrophion irregulare Sand-scoured false kelp Pterygophora californica Woody-stemmed kelp, Stalked kelp, Walking kelp Ralfsia sp. Silvetia compressa Rockweed Stephanocystis osmundacea Page 1 of 4 Red Algae or Rhodophyta Genus/Species Common Name Ahnfeltia fastigiata Bushy Ahnfelt's seaweed Ahnfeltiopsis linearis Anisocladella pacifica Bangia sp. Bossiella dichotoma Bossiella -

Identification of Conifer Trees in Iowa This Publication Is Designed to Help Identify the Most Common Trees Found in Iowa
Identification of Conifer Trees in Iowa This publication is designed to help identify the most common trees found in Iowa. It is based on vegetative characteristics including leaves, fruit, and bark. It is neither complete nor without possible oversights. Separate species are grouped by similar characteristics, mainly based on type and arrangement of leaves. These groups are; awl- or scale- like needles; single needles, flattened with rounded tips; single needles, square in cross section, with pointed tips; and needles in bundles or fasticles of two or more. Remember, vegetative character- istics are quite variable; use more than one specimen for comparison. Awl- or scale-like needles Juniperus Virginiana Eastern Red Cedar Leaves are dark green; leaves are both awl- and scale-like; cone is dark blue and berry-like. Thuja occidentalis Northern White Cedar Leaves are flattened and only of the scale type; cones have 4-6 scales; foliage is light green. Juniperus communis Common Juniper Leaves are awl shaped; cone is dark blue and berry-like. Pm-1383 | May 1996 Single needles, flattened with rounded tips Pseudotsuga menziesii Douglas Fir Needles occur on raised pegs; 3/4-11/4 inches in length; cones have 3-pointed bracts between the cone scales. Abies balsamea Abies concolor Balsam Fir White (Concolor) Fir Needles are blunt and notched at Needles are somewhat pointed, the tip; 3/4-11/2 inches in length. curved towards the branch top and 11/2-3 inches in length; silver green in color. Single needles, Picea abies Norway Spruce square in cross Needles are 1/2-1 inch long; section, with needles are dark green; foliage appears to droop or weep; cone pointed tips is 4-7 inches long. -

The Moss-Back Alga (Cladophorophyceae, Chlorophyta) on Two Species of Freshwater Turtles in the Kimberleys
Telopea 12(2) 279–284 The moss-back alga (Cladophorophyceae, Chlorophyta) on two species of freshwater turtles in the Kimberleys Stephen Skinner1,2, Nancy FitzSimmons3 and Timothy J. Entwisle1 1National Herbarium of New South Wales, Mrs Macquaries Road, Sydney NSW 2000 Australia 2Southern ACT Catchment Group Inc., PO Box 2056, Kambah, ACT Author for correspondence: [email protected] 3Institute for Applied Ecology, School of Resource, Environmental & Heritage Sciences, University of Canberra, Canberra, ACT 2601, Australia Abstract The range of the Australian freshwater alga Basicladia ramulosa Ducker is extended, both in its turtle hosts (Chelodina burrungandjii Thomson et al.; Emydura australis (Grey)) and in geography, to tropical northern Western Australia. Along with further morphological observations, sporangia are described for the first time in this taxon. Introduction Moss-back turtles (Fig. 1) have fascinated biologists for many years. While the carapace of a potentially amphibious turtle would be a challenging habitat for most aquatic organisms, it is perhaps surprising there are only a handful of attached algae reported from such sites. Edgren et al. (1953) detailed the range of host turtles then known in North America and the range of epizoic algae that included Rhizoclonium and Cladophora. Two further genera in the Cladophoraceae are the only macroalgae widely reported on turtle carapaces: the prostrate, spreading, endozoic (and possibly disease causing) Dermatophyton radicans Peter, and species of the heterotrichous genus Basicladia, responsible for the name ‘moss-back’. In the United States, Basicladia is considered a small epizoic genus on turtles and water snails, of three to four taxa (John 2003). Hamilton (1948) described sexual reproduction in North American species of Basicladia involving the fusion of biflagellate zooids as is commonly the case in the Cladophoraceae. -

Dr. Mitch Pavao-Zuckerman Department of Ecology and Evolutionary Biology
Dr. Mitch Pavao-Zuckerman Department of Ecology and Evolutionary Biology 621621--82208220 mzuckermzucker@[email protected] OfficeOffice hours:hours: BiosciencesBiosciences WestWest 431431 WW andand FF 11--22 p.m.p.m. oror byby appointmentappointment Diversity of Plants Diversity of Plants (Fig 29.4) Chlorophyta Ancestral Alga Nontracheophytes Nonseed Tracheophytes Gymnosperms The Transition to Life on Land Angiosperms The Vascular Plants The Seed Plants The Flowering Plants Monophyly • Monophyletic group – includes the most recent common ancestor and all decendents • These are NOT monophyletic: GreenGreen PlantsPlants ((viridiphytesviridiphytes)) areare aa monophyleticmonophyletic groupgroup • Green Plants include the Chlorophytes (green algae) • Other green algae • and the land plants EmbryophytesEmbryophytes (Land(Land Plants)Plants) Land Plants are also a monophyletic group • Photosynthetic eukaryotes that use chlorophyll a and b and store carbohydrates starch • Resting embryo with placental connection to the parent. The Conquest of the Land HistoryHistory ofof plantsplants onon landland •• 500500 myamya -- aa fewfew algaealgae andand lichens.lichens. •• ByBy 460460 myamya -- primitiveprimitive LandLand PlantsPlants,, •• ByBy 425425 myamya -- EarlyEarly VascularVascular PlantsPlants werewere commoncommon •• HowHow diddid itit happen?happen? •• Obstacles?Obstacles? Reconstruction Fossil The Conquest of the Land EarlyEarly innovationsinnovations inin landland plantplant evolution:evolution: 1.1. cuticlecuticle (waxy(waxy -
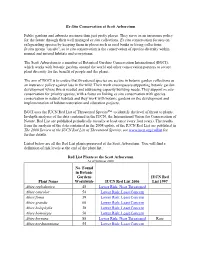
IUCN Red List of Threatened Species™ to Identify the Level of Threat to Plants
Ex-Situ Conservation at Scott Arboretum Public gardens and arboreta are more than just pretty places. They serve as an insurance policy for the future through their well managed ex situ collections. Ex situ conservation focuses on safeguarding species by keeping them in places such as seed banks or living collections. In situ means "on site", so in situ conservation is the conservation of species diversity within normal and natural habitats and ecosystems. The Scott Arboretum is a member of Botanical Gardens Conservation International (BGCI), which works with botanic gardens around the world and other conservation partners to secure plant diversity for the benefit of people and the planet. The aim of BGCI is to ensure that threatened species are secure in botanic garden collections as an insurance policy against loss in the wild. Their work encompasses supporting botanic garden development where this is needed and addressing capacity building needs. They support ex situ conservation for priority species, with a focus on linking ex situ conservation with species conservation in natural habitats and they work with botanic gardens on the development and implementation of habitat restoration and education projects. BGCI uses the IUCN Red List of Threatened Species™ to identify the level of threat to plants. In-depth analyses of the data contained in the IUCN, the International Union for Conservation of Nature, Red List are published periodically (usually at least once every four years). The results from the analysis of the data contained in the 2008 update of the IUCN Red List are published in The 2008 Review of the IUCN Red List of Threatened Species; see www.iucn.org/redlist for further details. -

The Marine Species of Cladophora (Chlorophyta) from the South African East Coast
NovaHedwigia 76 1—2 45—82 Stuttgart, Februar 2003 The marine species of Cladophora (Chlorophyta) from the South African East Coast by F. Leliaert and E. Coppejans Research Group Phycology, Department of Biology, Ghent University, Krijgslaan 281, S8 B-9000 Ghent, Belgium E-mails: [email protected] and [email protected] With 16 figures and 5 tables Leliaert, F. & E. Coppejans (2003): The marine species of Cladophora (Chlorophyta) from the South African East Coast. - Nova Hedwigia 76: 45-82. Abstract: Twelve species of the genus Cladophora occur along the South African East Coast. Detailed descriptions and illustrations are presented. Four species are recorded for the first time in South Africa: C. catenata , C. vagabunda , C. horii and C. dotyana; the last two are also new records for the Indian Ocean. A comparison of the South African C. rugulosa specimens with specimens of C. prolifera from South Africa and other regions have shown that these species are not synonymous as previously considered, leading to the resurrection of C. rugulosa which is probably a South African endemic. Key words: Cladophora, C. catenata , C. dotyana, C. horii, C. prolifera , C. rugulosa , C. vagabunda , South Africa, KwaZulu-Natal. Introduction Cladophora Kützing is one of the largest green-algal genera and has a worldwide distribution. Within the class Cladophorophyceae the genus Cladophora is characterized by its simple thallus architecture: branched, uniseriate filaments of multinucleate cells. Eleven different architectural types (sections) are distinguished in the genus (van den Hoek 1963, 1982; van den Hoek & Chihara 2000). Recent studies based on morphological and molecular data have proven that Cladophora is polyphyletic (van den Hoek 1982; Bakker et al. -

Apparent Hybridisation of Firecrest and Goldcrest F
Apparent hybridisation of Firecrest and Goldcrest F. K. Cobb From 20th to 29th June 1974, a male Firecrest Regulus ignicapillus was seen regularly, singing strongly but evidently without a mate, in a wood in east Suffolk. The area had not been visited for some time before 20th June, so that it is not known how long he had been present. The wood covers some 10 ha and is mainly deciduous, com prised of oaks Quercus robur, sycamores Acer pseudoplatanus, and silver birches Betula pendula; there is also, however, a scatter of European larches Larix decidua and Scots pines Pinus sylvestris, with an occasional Norway spruce Picea abies. Apart from the silver birches, most are mature trees. The Firecrest sang usually from any one of about a dozen Scots pines scattered over half to three-quarters of a hectare. There was also a single Norway spruce some 18-20 metres high in this area, which was sometimes used as a song post, but the bird showed no preference for it over the Scots pines. He fed mainly in the surround ing deciduous trees, but was never heard to sing from them. The possibility of an incubating female was considered, but, as the male showed no preference for any particular tree, this was thought unlikely. Then, on 30th June, G. J. Jobson saw the Firecrest with another Regulus in the Norway spruce and, later that day, D. J. Pearson and J. G. Rolfe watched this second bird carrying a feather in the same tree. No one obtained good views of it, but, not unnaturally, all assumed that it was a female Firecrest. -

Preservative Treatment of Scots Pine and Norway Spruce
Preservative treatment of Scots pine and Norway spruce Ron Rhatigan Camille Freitag Silham El-Kasmi Jeffrey J. Morrell✳ Abstract The ability to treat Scots pine (Pinus sylvestris) and Norway spruce (Picea abies) with oilborne copper-8-quinolinolate or with waterborne chromated copper arsenate, ammoniacal copper zinc arsenate, or ammoniacal copper quaternary was assessed using commercial treatment facilities in the Pacific Northwest. In general, Scots pine was more easily treated than was Norway spruce, al- though neither species could be treated to the standards of the American Wood-Preservers’Association for dimension lumber without incising. Treatment was better with ammonia-based solutions, reflecting the ability of these systems to overcome the effects of pit as- piration and encrustation, and their ability to swell the wood to improve permeability. The results indicate that successful treatment of both species will require the use of incising. In addition, further research will be required to identify suitable schedules for success- fully treating Norway spruce with oilborne copper-8-quinolinolate. he past decade has witnessed dra- other applications where preservative data for inclusion in the appropriate matic changes in the patterns of world treatment will be essential for achieving American Wood-Preservers’ Associa- timber trade. Countries that formerly adequate performance, it is important to tion Standards (AWPA) (1). This report were net exporters of timber now find ensure that they meet treatability stan- describes an evaluation of commercial themselves importing an ever-increasing dards, in addition to the more obvious treatment of Scots pine and Norway variety of wood species to meet domestic code requirements for strength. -

Monitoring of Biofouling Communities in a Portuguese Port Using a Combined Morphological and Metabarcoding Approach Joana Azevedo 1,2,3, Jorge T
www.nature.com/scientificreports OPEN Monitoring of biofouling communities in a Portuguese port using a combined morphological and metabarcoding approach Joana Azevedo 1,2,3, Jorge T. Antunes1,2,3, André M. Machado 1, Vitor Vasconcelos1,2, Pedro N. Leão 1* & Elsa Froufe 1* Marine biofouling remains an unsolved problem with a serious economic impact on several marine associated industries and constitutes a major vector for the spread of non-indigenous species (NIS). The implementation of biofouling monitoring programs allows for better fouling management and also for the early identifcation of NIS. However, few monitoring studies have used recent methods, such as metabarcoding, that can signifcantly enhance the detection of those species. Here, we employed monthly monitoring of biofouling growth on stainless steel plates in the Atlantic Port of Leixões (Northern Portugal), over one year to test the efect of commercial anti-corrosion paint in the communities. Fouling organisms were identifed by combining morpho-taxonomy identifcation with community DNA metabarcoding using multiple markers (16S rRNA, 18S rRNA, 23S rRNA, and COI genes). The dominant colonizers found at this location were hard foulers, namely barnacles and mussels, while other groups of organisms such as cnidarians, bryozoans, and ascidians were also abundant. Regarding the temporal dynamics of the fouling communities, there was a progressive increase in the colonization of cyanobacteria, green algae, and red algae during the sampled period with the replacement of less abundant groups. The tested anticorrosion paint demonstrated to have a signifcant prevention efect against the biofouling community resulting in a biomass reduction. Our study also reports, for the frst time, 29 NIS in this port, substantiating the need for the implementation of recurring biofouling monitoring programs in ports and harbours.