How to Submit Projects Into the Boston Strong Workflow Management System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microsoft Sharepoint 2013 Inside out Pdf, Epub, Ebook
MICROSOFT SHAREPOINT 2013 INSIDE OUT PDF, EPUB, EBOOK Darvish Shadravan,Penelope Coventry,Thomas Resing,Christina Wheeler | 904 pages | 28 Jun 2013 | Microsoft Press,U.S. | 9780735666993 | English | Redmond, United States Microsoft SharePoint 2013 Inside Out PDF Book On a practical note, the clarity of many of the screen shots in the book is disappointing and can make them hard to read. This supremely organized reference packs hundreds of timesaving solutions, troubleshooting techniques, and workarounds. Discover how the experts tackle SharePoint design, deployment, configuration, and management--and challenge yourself to new levels of mastery. This eBook requires no passwords or activation to read. At just over pages, this book is not for the faint-hearted, but if you are interested in becoming a SharePoint power user then this may be the book for you. Learn More. Cookie Settings New Signature uses "Required Cookies" to run our website, "Functional Cookies" used by third parties to personalise marketing, including social media features. Email Address. There are also quite a few cross references to other chapters if the current chapter does depend on knowledge presented in another part of the book. There are also quite a few cross references to other chapters if the current chapter does depend on knowledge presented in another part of the book. Testimonials We love transforming our customers businesses, take a look at what they have to say about New Signature. Targeting Cookies are used to capture user information in order for New Signature to deliver better user experiences. Lastly you will be exposed to creating and configuring Federated Search Sources. -

Infopath 2010 Project Tracker Case Study
InfoPath 2010 Project Tracker Case Study Microsoft InfoPath 2010 Project Tracker Global Manufacturing Company Te chnology InfoPath™ 2010 , SharePoint™ 2010, Web Services, Custom Web Parts, SharePoint Reporting Services, Dashboards Problem The client had multiple projects across the globe, and were using inefficient Microsoft Excel spreadsheets to track the budgets and financial progress of these projects. Not only were Excel spreadsheets being used exclusively, they were also being stored on multiple shared drives. Management had no way to easily access documents or assess performance on projects. Reports were difficult to generate and real-time information was extremely difficult to obtain. Solution ConvergePoint was approached to convert the existing excel forms into a robust SharePoint 2010 site using Microsoft InfoPath 2010 forms to aid budget tracking and Web Services for updating multiple data sources. The ConvergePoint InfoPath consulting team began with a business analysis of all existing spreadsheets that were being used by the client. Our team was able to determine the business value and organizational structure of the existing spreadsheets as well as proposed workflow modifications that would be preferred in the conversion. Continued ConvergePoint.com 888.484.8048 InfoPath 2010 Project Tracker Case Study Microsoft InfoPath 2010 Project Tracker Global Manufacturing Company Solution One of the areas given specific importance was that the InfoPath forms Continued were all exclusively web-based and could be securely accessed from any compatible browser. To provide access from 3rd party platforms, information would be stored in the InfoPath forms on the SharePoint site and also in a SQL database server. A SharePoint site was created as a library to store the InfoPath forms and resulting data. -

Microsoft's Solution: Infopath
04_579487 ch01.qxd 2/3/05 8:38 PM Page 1 1 InfoPath — The Journey Begins InfoPath is a new journey for both users and developers. If you are like many people, you may have many questions about just what InfoPath is and how it will help you in your work. That is what this chapter is all about. In it you will: ❑ Learn what InfoPath is, and how it can be used for various solutions. ❑ Read about different ways to connect data to InfoPath. ❑ Understand deployment requirements of InfoPath forms. ❑ Take a look at a typical InfoPath form. What Is InfoPath, and How Can It Be Used? InfoPath is a forms management tool that enables you to create forms and publish them in a num- ber of different ways. InfoPath enables you to use forms that are attached to various types of exist- ing data, and to use data that is self-contained within the form. Before going into more detail, take a look at what the COPYRIGHTEDproblem was before InfoPath came MATERIAL about. The Challenge of Forms Management Over the last decade there have been a number of attempts, both inside and outside of Microsoft, to enable users to create forms quickly, yet responsibly, to work with data. You can see this with database systems such as Access, and the form tools in Word and Excel. However, these tools have either come up short in helping users control and share their data, or they are too confusing to use to create forms that can handle data made up of more single-table solutions. -

Technical Reference for Microsoft Sharepoint Server 2010
Technical reference for Microsoft SharePoint Server 2010 Microsoft Corporation Published: May 2011 Author: Microsoft Office System and Servers Team ([email protected]) Abstract This book contains technical information about the Microsoft SharePoint Server 2010 provider for Windows PowerShell and other helpful reference information about general settings, security, and tools. The audiences for this book include application specialists, line-of-business application specialists, and IT administrators who work with SharePoint Server 2010. The content in this book is a copy of selected content in the SharePoint Server 2010 technical library (http://go.microsoft.com/fwlink/?LinkId=181463) as of the publication date. For the most current content, see the technical library on the Web. This document is provided “as-is”. Information and views expressed in this document, including URL and other Internet Web site references, may change without notice. You bear the risk of using it. Some examples depicted herein are provided for illustration only and are fictitious. No real association or connection is intended or should be inferred. This document does not provide you with any legal rights to any intellectual property in any Microsoft product. You may copy and use this document for your internal, reference purposes. © 2011 Microsoft Corporation. All rights reserved. Microsoft, Access, Active Directory, Backstage, Excel, Groove, Hotmail, InfoPath, Internet Explorer, Outlook, PerformancePoint, PowerPoint, SharePoint, Silverlight, Windows, Windows Live, Windows Mobile, Windows PowerShell, Windows Server, and Windows Vista are either registered trademarks or trademarks of Microsoft Corporation in the United States and/or other countries. The information contained in this document represents the current view of Microsoft Corporation on the issues discussed as of the date of publication. -

Deployment Guide for Microsoft Office 2013 Preview
Deployment guide for Microsoft Office 2013 Preview Microsoft Corporation Published: July 2012 Author: Microsoft Office System and Servers Team ([email protected]) Abstract This book supports a preliminary release of Microsoft Office 2013 Preview and provides deployment instructions for Office 2013 Preview. The audiences for this book include application specialists, line-of- business application specialists, and IT administrators who are ready to deploy Office 2013 Preview. The content in this book is a copy of selected content in the Office 2013 Preview technical library as of the publication date. For the most current content, see the technical library on the web. This document is provided “as-is.” Information and views expressed in this document, including URL and other Internet website references, may change without notice. You bear the risk of using it. Some examples depicted herein are provided for illustration only and are fictitious. No real association or connection is intended or should be inferred. This document does not provide you with any legal rights to any intellectual property in any Microsoft product. You may copy and use this document for your internal, reference purposes. © 2012 Microsoft Corporation. All rights reserved. Microsoft, Access, Active Directory, Backstage, Bing, Excel, Groove, Hotmail, Hyper-V, InfoPath, Internet Explorer, Office 365, OneNote, Outlook, PerformancePoint, PowerPoint, SharePoint, Silverlight, SkyDrive, Visio, Visio Studio, Windows, Windows Live, Windows Mobile, Windows PowerShell, Windows Server, and Windows Vista are either registered trademarks or trademarks of Microsoft Corporation in the United States and/or other countries. The information contained in this document represents the current view of Microsoft Corporation on the issues discussed as of the date of publication. -

Notes De Cours Infopath 2003 À 2010
Notes de cours InfoPath 2003 à 2010 Vincent ISOZ, 2014-02-20 (V3.0 Revision 9) {oUUID 1.681} Vincent ISOZ Please consider the environment - do you really need to print this document!? Remarques: Pour qu'il soit utilisable d'une manière rationnelle et sans danger, ce support qui constitue un "super condensé" d'un exposé qui tiendrait très facilement sur plusieurs milliers de pages (voir les ouvrages de cette taille disponible sur le commerce) et qui constitue une suite logique de mes livres sur MS Access, le XML et SharePoint doit absolument être complété par de nombreuses notes et exposés oraux, au cours desquels les notions nouvelles sont présentées au moyen de situations concrètes et illustrées par de nombreux exemples dont le choix dépend essentiellement du déroulement de la formation afin d'exciter l'esprit critique des apprenants. Ce support correspond à une formation d'environ 15 jours à 6.5 heures par jour pour un groupe de 6 personnes. Il y a de nombreuses marques déposées qui sont nommées dans le présent support. Plutôt que d'utiliser le symbole du trademark sur chaque occurrence de marque nommée, j'ai choisi d'utiliser le nom seul uniquement dans un souci d'esthétique éditoriale (ce qui devrait aussi bénéficier au propriétaire de la marque), sans aucune intention de violer une quelconque réglementation ou législation. Pour terminer, je voudrais remercier ici les quelques collègues et clients qui ont bien voulu me faire part de leurs remarques pour améliorer le contenu de ce livre électronique. Il est cependant certain qu'il est encore perfectible sur de nombreux points. -

Infopath with Sharepoint® 2013 How-To
STEVEN MANN InfoPath with SharePoint ® 2013 HOW-TO 800 East 96th Street, Indianapolis, Indiana 46240 USA Infopath.indb i 7/12/13 9:53 AM InfoPath with SharePoint 2013 How-To Executive Editor Copyright © 2014 by Pearson Education, Inc. Greg Wiegand All rights reserved. No part of this book shall be reproduced, stored in a retrieval system, or transmitted by any means, electronic, mechanical, Executive Editor photocopying, recording, or otherwise, without written permission from the publisher. No patent liability is assumed with respect to the use of the Neil Rowe information contained herein. Although every precaution has been taken in Development Editor the preparation of this book, the publisher and author assume no respon- sibility for errors or omissions. Nor is any liability assumed for damages Mark Renfrow resulting from the use of the information contained herein. Managing Editor ISBN-13: 978-0-672-33694-2 ISBN-10: 0-672-33694-4 Sandra Schroeder Library of Congress Control Number: 2013944877 Project Editor Printed in the United States of America Seth Kerney First Printing: July 2013 Copy Editor Trademarks All terms mentioned in this book that are known to be trademarks or Keith Cline service marks have been appropriately capitalized. Pearson cannot attest to the accuracy of this information. Use of a term in this book should not Indexer be regarded as affecting the validity of any trademark or service mark. Erika Millen Warning and Disclaimer Proofreader Every effort has been made to make this book as complete and as accu- rate as possible, but no warranty or fitness is implied. -
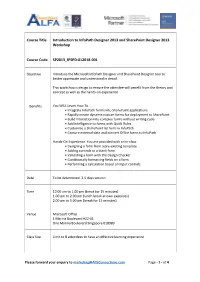
Course Title Introduction to Infopath Designer 2013 and Sharepoint Designer 2013 Workshop
Course Title Introduction to InfoPath Designer 2013 and SharePoint Designer 2013 Workshop Course Code SP2013_IPSPD-012018-001 Objective Introduce the Microsoft InfoPath Designer and SharePoint Designer tool to better appreciate and understand in detail. This workshop is design to ensure the attendee will benefit from the theory and concept as well as the hands-on experience. Benefits You WILL Learn How To … • Integrate InfoPath forms into SharePoint applications • Rapidly create dynamic custom forms for deployment to SharePoint • Build interaction into complex forms without writing code • Add intelligence to forms with Quick Rules • Customize a SharePoint list form in InfoPath • Capture external data and convert Office forms to InfoPath Hands-On Experience. You are provided with an in-class • Designing a form from a pre-existing template • Adding controls to a blank form • Validating a form with the Design Checker • Conditionally formatting fields on a form • Performing a calculation based on input controls Date To be determined. 2.5 days session Time 10.00 am to 1.00 pm (break for 15 minutes) 1.00 pm to 2.00 pm (lunch break at own expenses) 2.00 pm to 5.00 pm (break for 15 minutes) Venue Microsoft Office 1 Marina Boulevard #22-01 One Marina Boulevard Singapore 018989 Class Size Limit to 8 attendees to have an effective learning experience Please forward your enquiry to [email protected] Page - 1 - of 4 Technology Microsoft SharePoint Online or Microsoft SharePoint 2013 Notes to Attendee has to bring your own laptop (Windows 7 and above) for this Attendee workshop. Attendee has to ensure that the internet browser software is updated to the supported version with SharePoint 2013 server. -
![[MS-OCPROTO]: Office Client Protocols Overview](https://docslib.b-cdn.net/cover/8973/ms-ocproto-office-client-protocols-overview-1588973.webp)
[MS-OCPROTO]: Office Client Protocols Overview
[MS-OCPROTO]: Office Client Protocols Overview This document provides an overview of the protocols in the Microsoft® Office system. It is intended for use in conjunction with the Microsoft protocol technical documents, publicly available standard specifications, network programming art, and Microsoft Windows distributed systems concepts. It assumes that the reader is either familiar with the aforementioned material or has immediate access to it. This system does not require use of Microsoft programming tools or programming environments to implement the protocols within it. Implementers who have access to Microsoft programming tools and environments are free to take advantage of them. Intellectual Property Rights Notice for Open Specifications Documentation . Technical Documentation. Microsoft publishes Open Specifications documentation for protocols, file formats, languages, standards as well as overviews of the interaction among each of these technologies. Copyrights. This documentation is covered by Microsoft copyrights. Regardless of any other terms that are contained in the terms of use for the Microsoft website that hosts this documentation, you may make copies of it in order to develop implementations of the technologies described in the Open Specifications and may distribute portions of it in your implementations using these technologies or your documentation as necessary to properly document the implementation. You may also distribute in your implementation, with or without modification, any schema, IDL's, or code samples that are included in the documentation. This permission also applies to any documents that are referenced in the Open Specifications. No Trade Secrets. Microsoft does not claim any trade secret rights in this documentation. Patents. Microsoft has patents that may cover your implementations of the technologies described in the Open Specifications. -

ACTIVEDOCS Or MICROSOFT TECHNOLOGIES for DOCUMENT AUTOMATION
ACTIVEDOCS or MICROSOFT TECHNOLOGIES for DOCUMENT AUTOMATION Prepared by: Nick Chivers Director of Product Marketing Audience: ActiveDocs Evaluator Abstract: A comparison of the Document Automation capabilities of ActiveDocs and a collection of Microsoft® technologies. OVERLAND PARK LONDON AUCKLAND BRISBANE Southcreek Office Park 199 Bishopsgate Level 6, 27 Gillies Avenue 192 Ann Street 7301 West 129th Street London Newmarket, Auckland 1023 Brisbane, QLD 4000 Suite 160 EC2M 3TY Post: PO Box 289 Post: PO Box 604 Overland Park, KS 66213, USA United Kingdom Auckland 1140, New Zealand Paradise Point QLD 4216, Australia Ph +1 913 888 1999 Ph +44 20 3290 1788 Ph +64 9 520 5650 Ph +61 7 3040 6616 [email protected] | w ww.activedocs.com ACTIVEDOCS OPUS OR MICROSOFT TECHNOLOGIES FOR DOCUMENT AUTOMATION Copyright Information in this document is subject to change without notice. Companies, names, and data used in examples herein are fictitious unless otherwise noted. No part of this document may be reproduced or transmitted in any form or by any means, electronic or mechanical, for any purpose, without the express written permission of ActiveDocs Limited. Copyright © ActiveDocsTM Limited. All rights reserved. Microsoft is a registered trademark and Microsoft SQL Server, Microsoft Access, Microsoft Outlook, and Microsoft Windows are trademarks of Microsoft Corporation in the United States and/or other countries. Other product and company names herein may be the trademarks of their respective owners. Disclaimer: While ActiveDocs has taken care to ensure the accuracy and quality of this document, all content including fitness for a particular purpose are provided without any warranty whatsoever, either expressed or implied. -

Using Sharepoint's Infopath Feature to Generate Forms Creating A
SHAREPOINT PRACTICAL IT STRATEGIES FOR ENTERPRISE COLLABORATION /// OCTOBER 2008 I IMPLEMENTATION Using SharePoint’s InfoPath feature to generate forms SharePoint Server’s InfoPath can save money and time by streamlining the forms process. BY NICOLA YOUNG G GOVERNANCE Creating a SharePoint governance document Learn which issues to include in your SharePoint governance document to avoid problems down the road. BY BRIEN M. POSEY M MANAGEMENT Rookie mistakes to avoid during the implementation process From choosing the wrong installation to ignoring disaster recovery, beware of these SharePoint implementation blunders. BY SHAWN SHELL » EDITOR’SNOTE ++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ In search of the ‘paperless office’ BY CHRISTINE CASATELLI ++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Editor’s note FOR ENTERPRISES STILL STRIVING for the “paperless office,” Microsoft Office InfoPath might get you one step closer. I Office InfoPath is an application of Microsoft Office SharePoint Server 2007 that allows forms to be created and filled in using a Web browser. Users can Using SharePoint’s InfoPath feature publish these forms to a library and update them so everyone always uses the to generate forms latest version of each form. Web-based forms can save time and money, so why is InfoPath one of the G most underused SharePoint features? Creating a If the idea of writing custom code is a turn-off for IT folks, they needn’t worry. SharePoint governance SharePoint expert Nicola Young says that InfoPath uses a WYSIWYG forms document designer, so no programming is required. Young walks readers through the process of using an InfoPath template to design an online form in this month’s M cover story “Using SharePoint’s InfoPath feature to generate forms.” Rookie mistakes Is governance keeping you up at night? Then you’ve come to the right place. -

Microsoft Sharepoint 2013 Inside Out
Microsoft SharePoint 2013 Inside Out Darvish Shadravan Penelope Coventry Thomas Resing Christina Wheeler Copyright © 2013 Darvish Shadravan, PPP Consulting Ltd., Thomas Resing, Christina Wheeler All rights reserved. No part of the contents of this book may be reproduced or transmitted in any form or by any means without the written permission of the publisher. ISBN: 978-0-7356-6699-3 Third Printing: March 2015 Printed and bound in the United States of America. Microsoft Press books are available through booksellers and distributors worldwide. If you need support related to this book, email Microsoft Press Book Support at [email protected]. Please tell us what you think of this book at http://www.microsoft.com/learning/booksurvey. Microsoft and the trademarks listed at http://www.microsoft.com/about/legal/en/us/ IntellectualProperty/Trademarks/EN-US.aspx are trademarks of the Microsoft group of companies. All other marks are property of their respective owners. The example companies, organizations, products, domain names, email addresses, logos, people, places, and events depicted herein are fi ctitious. No association with any real company, organization, product, domain name, email address, logo, person, place, or event is intended or should be inferred. This book expresses the author’s views and opinions. The information contained in this book is provided without any express, statutory, or implied warranties. Neither the authors, Microsoft Corporation, nor its resellers, or distributors will be held liable for any damages caused or alleged to be caused either directly or indirectly by this book. Acquisitions and Development Editor: Kenyon Brown Production Editor: Rachel Steely Editorial Production: S4Carlisle, Inc.