Dicrocoelium Dendriticum Found in a Bronze Age Cemetery in Western
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Treasures from Near Eastern Looms
The Bowdoin College Library Treasures from Near Eastern Looms ERNEST H. ROBERTS BRUNSWICK, MAINE 1981 Bowdoin College Museum of Art Brunswick, Maine September 11, 1981 to November 22, 1981 The Textile Museum Washington, District of Columbia December 11, 1981 to February 6, 1982 Cover: Carpel Fnn>incni, Caucasian, Dagistan area, ca. 1850 Photographs by Robert H. Stillwell Design by Michael W. Mahan Printed byJ.S. McCarthy Co., Inc., Augusta, Maine Copyright © 1981 by Ernest H. Roberts Library of Congress Catalog Card Number: 81-68474 ISBN: 0-916606-02-3 Portions of this catalogue are reprinted in altered form from other publications. We are indebted to the following institutions for per- mission to use their material: to the Allen Memorial Art Museum, Oberlin, Ohio, for the chapter introductions and descriptions of plates 12, 19, 24, 28, 63, and 65, which appeared in "Catalogue of Islamic Carpets," Allen An Museum Bulletin 3 (1978-1979) by Ernest H. Roberts; to The Textile Museum, Washington, D.C., for glossary entries and drawings from "Definitions and Explana- tions," a section of Early Caucasian Ru^s by Charles Grant Ellis, published by that museum in 1975, and for the loan of the map which appears on page 61 of this book; to the Joslyn Art Museum, Omaha, Nebraska, for descriptions of plates 28, 35, 44, 57, and 67 from A Rich Inheritance: Oriental Ruj^s oj 19th and Early 20th Centuries, published by that museum in 1974; and to the Near Eastern Art Research Center, Inc., for the description of plate 68 from Islamic Carpets by Joseph V. -

US Covert Operations Toward Iran, February-November 1979
This article was downloaded by: [Tulane University] On: 05 January 2015, At: 09:36 Publisher: Routledge Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Middle Eastern Studies Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/fmes20 US Covert Operations toward Iran, February–November 1979: Was the CIA Trying to Overthrow the Islamic Regime? Mark Gasiorowski Published online: 01 Aug 2014. Click for updates To cite this article: Mark Gasiorowski (2015) US Covert Operations toward Iran, February–November 1979: Was the CIA Trying to Overthrow the Islamic Regime?, Middle Eastern Studies, 51:1, 115-135, DOI: 10.1080/00263206.2014.938643 To link to this article: http://dx.doi.org/10.1080/00263206.2014.938643 PLEASE SCROLL DOWN FOR ARTICLE Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information. Taylor and Francis shall not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of the Content. -

A Historical Sociology Approach to Iranian Nationalism (1921-1979): an Ir Perspective
A HISTORICAL SOCIOLOGY APPROACH TO IRANIAN NATIONALISM (1921-1979): AN IR PERSPECTIVE A THESIS SUBMITTED TO THE GRADUATE SCHOOL OF SOCIAL SCIENCES OF MIDDLE EAST TECHNICAL UNIVERSITY BY ZELAL ÖZDEMİR IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN THE PROGRAMME OF AREA STUDIES JUNE 2016 I hereby declare that all information in this document has been obtained and presented in accordance with academic rules and ethical conduct. I also declare that, as required by these rules and conduct, I have fully cited and referenced all material and results that are not original to this work. Name, Last name: Zelal Özdemir Signature : iii ABSTRACT A HISTORICAL SOCIOLOGY APPROACH TO IRANIAN NATIONALISM (1921-1979): AN IR PERSPECTIVE Özdemir, Zelal PhD, Programme of Area Studies Supervisor: Assoc. Prof. Dr. Ayça Ergun Özbolat Co-Supervisor: Prof. Dr. Meliha Altunışık June 2016, 232 pages This thesis explores the discourse of Iranian nationalism constructed by the Iranian state between 1921 and 1979. This study unravels the international connections of Iranian nationalism following a framework that sits at the intersection of Historical Sociology, Historical Sociology in International Relations and modernist approaches to nationalism. It argues that the creation and development of the definition of Iranian nationalism is interconnected with the modern state which is itself bound up with the international. In order to understand the nature of the meaning attached to being Iranian/Iranianness/Iraniyat on the part of the state we should look into the specifics of international-domestic interaction, historicise that process and search for multiple causes rather than one single cause. -

Iran (Persia) and Aryans Part - 4
INDIA (BHARAT) - IRAN (PERSIA) AND ARYANS PART - 4 Dr. Gaurav A. Vyas This book contains the rich History of India (Bharat) and Iran (Persia) Empire. There was a time when India and Iran was one land. This book is written by collecting information from various sources available on the internet. ROOTSHUNT 15, Mangalyam Society, Near Ocean Park, Nehrunagar, Ahmedabad – 380 015, Gujarat, BHARAT. M : 0091 – 98792 58523 / Web : www.rootshunt.com / E-mail : [email protected] Contents at a glance : PART - 1 1. Who were Aryans ............................................................................................................................ 1 2. Prehistory of Aryans ..................................................................................................................... 2 3. Aryans - 1 ............................................................................................................................................ 10 4. Aryans - 2 …............................………………….......................................................................................... 23 5. History of the Ancient Aryans: Outlined in Zoroastrian scriptures …….............. 28 6. Pre-Zoroastrian Aryan Religions ........................................................................................... 33 7. Evolution of Aryan worship ....................................................................................................... 45 8. Aryan homeland and neighboring lands in Avesta …...................……………........…....... 53 9. Western -

Iran (Persia) and Aryans Part - 6
INDIA (BHARAT) - IRAN (PERSIA) AND ARYANS PART - 6 Dr. Gaurav A. Vyas This book contains the rich History of India (Bharat) and Iran (Persia) Empire. There was a time when India and Iran was one land. This book is written by collecting information from various sources available on the internet. ROOTSHUNT 15, Mangalyam Society, Near Ocean Park, Nehrunagar, Ahmedabad – 380 015, Gujarat, BHARAT. M : 0091 – 98792 58523 / Web : www.rootshunt.com / E-mail : [email protected] Contents at a glance : PART - 1 1. Who were Aryans ............................................................................................................................ 1 2. Prehistory of Aryans ..................................................................................................................... 2 3. Aryans - 1 ............................................................................................................................................ 10 4. Aryans - 2 …............................………………….......................................................................................... 23 5. History of the Ancient Aryans: Outlined in Zoroastrian scriptures …….............. 28 6. Pre-Zoroastrian Aryan Religions ........................................................................................... 33 7. Evolution of Aryan worship ....................................................................................................... 45 8. Aryan homeland and neighboring lands in Avesta …...................……………........…....... 53 9. Western -

Palestine Issue Will Surely End in Favor of Islamic World: Abe Told a News Conference After His Coalition’S Victory in a Sunday Election for Parliament’S Upper House
WWW.TEHRANTIMES.COM I N T E R N A T I O N A L D A I L Y Pages Price 40,000 Rials 1.00 EURO 4.00 AED 39th year No.13443 Tuesday JULY 23, 2019 Mordad 1, 1398 Dhi Al Qada 20, 1440 Abdul Mahdi Iranian oil Iran beat Argentina Studios from Czech, Italy, holds talks with irreplaceable: at FIVB U21 World Ukraine join Iranian director Rouhani 2 Zanganeh 5 Championship 15 to make “Blue Land” 16 Iran arrests 17 professional Palestine issue will surely CIA spies TEHRAN — Iran’s Intelligence Ministry nuclear, infrastructural, military and announced on Monday that it had bro- cyber centers, where they collected ken up a CIA spy ring and arrested 17 classified information. professional spies, some of whom have “Some citizens were trapped by the been sentenced to death by the Judiciary. U.S. exploitation of their visa requests and end in favor of Islamic world According to the director general were encouraged to spy in exchange for of the Intelligence Ministry’s coun- receiving a visa,” the official said. “Some ter-espionage department, the spies others were blackmailed by the CIA due See page 2 were employed in sensitive and vital to their need of maintaining or extending state and private sectors in economic, their visas.” 3 Tehran’s tanker seizure arguments more convincing than London’s: Moscow TEHRAN — The Kremlin says Iran’s argu- moment of the arrest of a Panama-flagged ments for its recent seizure of a UK-flagged tanker carrying Iranian oil,” Russian oil tanker in the Strait of Hormuz were Deputy Foreign Minister Sergey Ryabkov more convincing than those of London. -
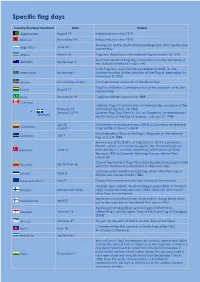
Specific Flag Days
Specific flag days Country/Territory/Continent Date Details Afghanistan August 19 Independence day, 1919. Albania November 28 Independence day, 1912. Anniversary of the death of Manuel Belgrano, who created the Argentina June 20 current flag. Aruba March 18 Flag day. Adoption of the national flag on March 18, 1976. Australian National Flag Day commemorates the first flying of Australia September 3 the Australian National Flag in 1901. State Flag Day, was officially established in 2009, for the Azerbaijan November 9 commemoration of the adoption of the Flag of Azerbaijan on November 9, 1918. Åland Last Sunday of April Commemorates adoption of the Åland flag Flag Day in Bolivia. Commemorates of the creation of the first August 17 Bolivia national flag. Brazil November 19 Flag Day in Brazil; adopted in 1889 Canada National Flag of Canada Day commemorates adoption of the February 15 Canadian flag, Feb. 15, 1965. January 21[4][5] Québec Flag Day (French: Jour du Drapeau) commemorates Quebec the first flying of the flag of Quebec, January 21, 1948. July 20 Declaration of Independence (1810) (Celebrated as National Colombia August 7 Day); Battle of Boyaca (1819) Dia di Bandera ("Day of the Flag"). Adoption of the national July 2 Curaçao flag on 2 July 1984. Anniversary of the Battle of Valdemar in 1219 in Lyndanisse, Estonia, where according to legend, the ("Dannebrog") fell Denmark June 15 from the sky. It is also the anniversary of the return of North Slesvig in 1920 to Denmark following the post-World War I plebiscite. "Day of the National Flag" ("Dia de la Bandera Nacional"). -

The Dynamics of Ethnic Identity in Iranian Azerbaijan Fereydoun Safizadeh
Journal of Pedagogy, Pluralism, and Practice Volume 5 | Issue 1 Article 6 Fall 2013 The Dynamics of Ethnic Identity in Iranian Azerbaijan Fereydoun Safizadeh Follow this and additional works at: https://digitalcommons.lesley.edu/jppp Recommended Citation Safizadeh, Fereydoun (2013) "The Dynamics of Ethnic Identity in Iranian Azerbaijan," Journal of Pedagogy, Pluralism, and Practice: Vol. 5 : Iss. 1 , Article 6. Available at: https://digitalcommons.lesley.edu/jppp/vol5/iss1/6 This Article is brought to you for free and open access by DigitalCommons@Lesley. It has been accepted for inclusion in Journal of Pedagogy, Pluralism, and Practice by an authorized editor of DigitalCommons@Lesley. For more information, please contact [email protected]. Safizadeh: The Dynamics of Ethnic Identity in Iranian Azerbaijan 55 The Dynamics of Ethnic Identity in Iranian Azerbaijan Fereydoun Safizadeh Introduction The population in Iran has doubled since the 1979 Islamic Revolution, and today it stands at nearly 75 million. Iran has a multiethnic, multi-tribal, multilingual, multicultural and multi- religious society. In addition to the historic Persian-Iranian identity, the population has a multi-layered and multi-faceted cultural, linguistic, tribal, religious, regional identification and affiliations. The diversity of society harks back to an earlier era, prior to the imposition of either/or dichotomous principles of ethnic nationalism at the turn of 20th century in Europe and elsewhere. Today, this dichotomous envisioning of society is best exemplified by the figure such as the one below where you have a highly simplified chart representing the composition of the society that is ethnographically unable to capture any of the multi- faceted and the multi-layered dimensions of group identity in Iran. -

Kaşkay Têrkæesinde Sayi Sézcêkleri: Şiraz Ve Æevresinden Érnekler
ERENOĞLU ATAĠZĠ, D. (2017). KaĢkay Türkçesinde Sayı Sözcükleri: ġiraz ve Çevresinden Örnekler. Uluslararası Türkçe Edebiyat Kültür Eğitim Dergisi, 6(2), 865-877. Uluslararası Türkçe Edebiyat Kültür Eğitim Dergisi Sayı: 6/2 2017 s. 865-877, TÜRKĠYE KAŞKAY TÊRKÆESİNDE SAYI SÉZCÊKLERİ: ŞİRAZ VE ÆEVRESİNDEN ÉRNEKLER Dilek ERENOĞLU ATAİZİ Geliş Tarihi: Mayıs, 2017 Kabul Tarihi: Haziran, 2017 Éz Sayılar dillerin temel söz varlığıdır. Dilin varlığı, sayı sisteminin varlığı ile ölçülür. Türk sayı sistemleri Göktürkçe kökenlidir. Türkçenin Oğuz coğrafyasında kullanılan sayı kelimeleri de bu temel kaynaktan gelmektedir. Bir Oğuz kolu olan yarı göçebe KaĢkay Türkleri Ġran sınırları içinde, çoğunlukla ġiraz ve çevresinde yaĢamaktadırlar. Ġki dilli KaĢkay halkı, sayı kelimelerini kullanmak bakımından diğer Türk Ģivelerinden ayrılmaz, bununla birlikte Farsçanın etkileri de görmezden gelinecek kadar az değildir. Bu yazıda Türk Dil Kurumu Projesi olarak hazırlanmıĢ olan KaĢkay Türklerinin Dili adlı çalıĢmanın derlemelerinden bir kısmına dayanarak KaĢkay Türklerinin dilindeki sayılar incelenecektir. Anahtar Sözcükler: Sayı, sayı kelimeleri, KaĢkay Türkleri, KaĢkay Türkçesi, Ġran Türkleri, yarı göçebe. NUMBER WORDS OF QASHQAI: EXAMPLES FROM SHIRAZ AND ITS SURROUNDINGS Abstract Numbers are the basic word entities of the languages. The entity of the language is measured with the number system. Turkish number systems have Gokturkish roots. Number words of Turkish which are used in Oghuz geography also come from this basic source. Semi-nomadic Qashqai Turks, which is an Oghuz branch, lives within the borders of Iran, mostly in the Shiraz and its surroundings. Qashqai people who are bilingual are not separated from other Turkish dialects in terms of using number words. In addition to this, the influence of Farsi cannot be underestimated. -

Qashqai Nomads Mean Authenticity
Art & Culture February 27, 2018 3 This Day in History Qashqai Nomads Mean (February 27) Authenticity Today is Tuesday; 8th of the Iranian month of Esfand 1396 solar hijri; corresponding to 10th of the Islamic month of Jamadi as-Sani 1439 lunar hijri; and February 27, TEHRAN (IFP) -- The unique and innocent faces of tribal Qashqai women, men and chil- 2018, of the Christian Gregorian Calendar. dren, who always pass through the mountains and plains, have different expressions. The 1746 solar years ago, on this day in 272 AD, Constantine I, who imposed the small lines on the face to the bristling of a woman’s hair, each narrate the story of the days Pauline Creed on the Roman Empire, was born. His father, Flavius Valerius Constantius, was an army officer, who on becoming deputy emperor of the west in of nomadic life. 293, sent Constantine to the east as a military tribune to the emperors Diocletian and The traditional nomadic Qashqai people travel with their flocks twice a year to and from the Galerius – notorious for their persecution of the monotheist followers of Prophet summer highland pastures north of Shiraz roughly 480 km or 300 miles south to the winter pas- Jesus and those who later came to be known as Christians. In 305, his father was raised to the rank of Augustus, or senior western emperor, and Constantine was tures on lower and warmer lands near the Persian Gulf, to the southwest of Shiraz. They live in recalled to the west to campaign in Britannia. Acclaimed as emperor by the army on black tents that are made of goat wool. -

Taghi Farvar – 1942-2018
Promoting the appropriate recognition of, and support to, the territories and areas conserved by indigenous peoples and local communities (ICCAs) www.iccaconsortium.org Dr. Mohammad Taghi Farvar – 1942-2018 Born in 1942, M. Taghi Farvar was heir of Shahsevan indigenous pastoralists in Iranian Azerbaijan. And nomad he remained all his life, working in all continents and being at ease with people from diverse languages, cultures, religions and world views. As President of the ICCA Consortium, he was our inspirer and motivator in chief. Many of us dedicate time and energy to the Consortium beyond the call of duty because of Taghi, because we met him, worked with him, were moved and influenced by him. Taghi led at least three international movements from within—all tied by a unifying theme. The first movement Taghi brought to the fore is the one that pioneered the critique of development and opened countless eyes to the unintended consequences for environment and health of major “development” initiatives (dams, ports, roads, urbanization, deforestation, large scale agribusiness and extractive industries…). He did so by doing extensive research and organising conferences while he was working on his PhD with Barry Commoner in the 1960s and early 1970s. This was one of the earliest ever interdisciplinary PhDs, linking anthropology and environmental sciences (his thesis, defended at Washington University, investigated DDT residues in mothers’ milk among plantation workers in Guatemala). It was around that time that Taghi took part in the civil rights movement in the USA, was assigned to share rooms with African-Americans in the university (the USA was an apartheid country and he was an Iranian, after all), was badly beaten by racist thugs, and edited and published the book The Careless Technology: Ecology and International Development (New York, Doubleday/Natural History Press, Conservation Foundation and Centre for the Biology of Natural Systems, 1030pp, 1972), which remains a classic. -
Iran Case File (April 2019)
IRAN CASE FILE October 2020 RASANAH International Institute for Iranian Studies Contact us [email protected] +966112166696 The Executive Summary .............................................................3 Internal Affairs .........................................................................7 The Ideological File ......................................................................... 8 I. Marja al-Haydari and the Attempts at Reform ..................................... 8 II. The Position of the Iranian Government: Excluding and Defaming Haydari........................................................... 9 III. Iraq Gets Embroiled in the Crisis ......................................................10 The Political File ............................................................................12 I. The Protests ......................................................................................12 II. The Iranian Government’s Reaction Towards the Azeri Protests ........13 III. Iranian National Security in Light of the Northwestern Borders Turning Into a Threatening Hotspot .............14 The Economic File ..........................................................................18 I. Iran’s Public Debt and Causes ............................................................18 II. Reading the Debt to GDP Ratio .........................................................19 III. The Future Trends of Iranian Government Debt ...............................21 The Military File.............................................................................24