Journal Club Pyrrolidine Synthesis Via C–H Bond Amination
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Origin of the Peculiarities of the Vietnamese Alphabet André-Georges Haudricourt
The origin of the peculiarities of the Vietnamese alphabet André-Georges Haudricourt To cite this version: André-Georges Haudricourt. The origin of the peculiarities of the Vietnamese alphabet. Mon-Khmer Studies, 2010, 39, pp.89-104. halshs-00918824v2 HAL Id: halshs-00918824 https://halshs.archives-ouvertes.fr/halshs-00918824v2 Submitted on 17 Dec 2013 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Published in Mon-Khmer Studies 39. 89–104 (2010). The origin of the peculiarities of the Vietnamese alphabet by André-Georges Haudricourt Translated by Alexis Michaud, LACITO-CNRS, France Originally published as: L’origine des particularités de l’alphabet vietnamien, Dân Việt Nam 3:61-68, 1949. Translator’s foreword André-Georges Haudricourt’s contribution to Southeast Asian studies is internationally acknowledged, witness the Haudricourt Festschrift (Suriya, Thomas and Suwilai 1985). However, many of Haudricourt’s works are not yet available to the English-reading public. A volume of the most important papers by André-Georges Haudricourt, translated by an international team of specialists, is currently in preparation. Its aim is to share with the English- speaking academic community Haudricourt’s seminal publications, many of which address issues in Southeast Asian languages, linguistics and social anthropology. -

1 Curl and Divergence
Sections 15.5-15.8: Divergence, Curl, Surface Integrals, Stokes' and Divergence Theorems Reeve Garrett 1 Curl and Divergence Definition 1.1 Let F = hf; g; hi be a differentiable vector field defined on a region D of R3. Then, the divergence of F on D is @ @ @ div F := r · F = ; ; · hf; g; hi = f + g + h ; @x @y @z x y z @ @ @ where r = h @x ; @y ; @z i is the del operator. If div F = 0, we say that F is source free. Note that these definitions (divergence and source free) completely agrees with their 2D analogues in 15.4. Theorem 1.2 Suppose that F is a radial vector field, i.e. if r = hx; y; zi, then for some real number p, r hx;y;zi 3−p F = jrjp = (x2+y2+z2)p=2 , then div F = jrjp . Theorem 1.3 Let F = hf; g; hi be a differentiable vector field defined on a region D of R3. Then, the curl of F on D is curl F := r × F = hhy − gz; fz − hx; gx − fyi: If curl F = 0, then we say F is irrotational. Note that gx − fy is the 2D curl as defined in section 15.4. Therefore, if we fix a point (a; b; c), [gx − fy](a; b; c), measures the rotation of F at the point (a; b; c) in the plane z = c. Similarly, [hy − gz](a; b; c) measures the rotation of F in the plane x = a at (a; b; c), and [fz − hx](a; b; c) measures the rotation of F in the plane y = b at the point (a; b; c). -

Ffontiau Cymraeg
This publication is available in other languages and formats on request. Mae'r cyhoeddiad hwn ar gael mewn ieithoedd a fformatau eraill ar gais. [email protected] www.caerphilly.gov.uk/equalities How to type Accented Characters This guidance document has been produced to provide practical help when typing letters or circulars, or when designing posters or flyers so that getting accents on various letters when typing is made easier. The guide should be used alongside the Council’s Guidance on Equalities in Designing and Printing. Please note this is for PCs only and will not work on Macs. Firstly, on your keyboard make sure the Num Lock is switched on, or the codes shown in this document won’t work (this button is found above the numeric keypad on the right of your keyboard). By pressing the ALT key (to the left of the space bar), holding it down and then entering a certain sequence of numbers on the numeric keypad, it's very easy to get almost any accented character you want. For example, to get the letter “ô”, press and hold the ALT key, type in the code 0 2 4 4, then release the ALT key. The number sequences shown from page 3 onwards work in most fonts in order to get an accent over “a, e, i, o, u”, the vowels in the English alphabet. In other languages, for example in French, the letter "c" can be accented and in Spanish, "n" can be accented too. Many other languages have accents on consonants as well as vowels. -

Medical Terminology Abbreviations Medical Terminology Abbreviations
34 MEDICAL TERMINOLOGY ABBREVIATIONS MEDICAL TERMINOLOGY ABBREVIATIONS The following list contains some of the most common abbreviations found in medical records. Please note that in medical terminology, the capitalization of letters bears significance as to the meaning of certain terms, and is often used to distinguish terms with similar acronyms. @—at A & P—anatomy and physiology ab—abortion abd—abdominal ABG—arterial blood gas a.c.—before meals ac & cl—acetest and clinitest ACLS—advanced cardiac life support AD—right ear ADL—activities of daily living ad lib—as desired adm—admission afeb—afebrile, no fever AFB—acid-fast bacillus AKA—above the knee alb—albumin alt dieb—alternate days (every other day) am—morning AMA—against medical advice amal—amalgam amb—ambulate, walk AMI—acute myocardial infarction amt—amount ANS—automatic nervous system ant—anterior AOx3—alert and oriented to person, time, and place Ap—apical AP—apical pulse approx—approximately aq—aqueous ARDS—acute respiratory distress syndrome AS—left ear ASA—aspirin asap (ASAP)—as soon as possible as tol—as tolerated ATD—admission, transfer, discharge AU—both ears Ax—axillary BE—barium enema bid—twice a day bil, bilateral—both sides BK—below knee BKA—below the knee amputation bl—blood bl wk—blood work BLS—basic life support BM—bowel movement BOW—bag of waters B/P—blood pressure bpm—beats per minute BR—bed rest MEDICAL TERMINOLOGY ABBREVIATIONS 35 BRP—bathroom privileges BS—breath sounds BSI—body substance isolation BSO—bilateral salpingo-oophorectomy BUN—blood, urea, nitrogen -

Proposal for Generation Panel for Latin Script Label Generation Ruleset for the Root Zone
Generation Panel for Latin Script Label Generation Ruleset for the Root Zone Proposal for Generation Panel for Latin Script Label Generation Ruleset for the Root Zone Table of Contents 1. General Information 2 1.1 Use of Latin Script characters in domain names 3 1.2 Target Script for the Proposed Generation Panel 4 1.2.1 Diacritics 5 1.3 Countries with significant user communities using Latin script 6 2. Proposed Initial Composition of the Panel and Relationship with Past Work or Working Groups 7 3. Work Plan 13 3.1 Suggested Timeline with Significant Milestones 13 3.2 Sources for funding travel and logistics 16 3.3 Need for ICANN provided advisors 17 4. References 17 1 Generation Panel for Latin Script Label Generation Ruleset for the Root Zone 1. General Information The Latin script1 or Roman script is a major writing system of the world today, and the most widely used in terms of number of languages and number of speakers, with circa 70% of the world’s readers and writers making use of this script2 (Wikipedia). Historically, it is derived from the Greek alphabet, as is the Cyrillic script. The Greek alphabet is in turn derived from the Phoenician alphabet which dates to the mid-11th century BC and is itself based on older scripts. This explains why Latin, Cyrillic and Greek share some letters, which may become relevant to the ruleset in the form of cross-script variants. The Latin alphabet itself originated in Italy in the 7th Century BC. The original alphabet contained 21 upper case only letters: A, B, C, D, E, F, Z, H, I, K, L, M, N, O, P, Q, R, S, T, V and X. -

Model C(G)-310 100-Pair Connector
Features ■ Front-facing confi guration - easy access to protector modules, jumper fi eld and test fi eld ■ Increased density - confi guration increases pair density by 25 percent when compared to traditional 303-type connector blocks ■ Industry-standard design - Accepts all standard 303-type protector modules and testing equipment ■ Integral fanning strip helps maintain neat and orderly wiring Model C(G)-310 100-Pair Connector Bourns® C(G)-310 Connector is intended for applications where protector pair density and front-facing protector modules, test fi eld and jumper fi eld are important. It is ideal for “protector only” frames where the jumpering between the switching equipment and the connector is done on separate, intermediate distributing frames. This 362 mm high (14.25 inch) connector maintains the basic features of the 303 connector while increasing the protected pair density by 25 percent compared to the C(G)-303. The C(G)-310 connector consists of a one-piece, fl ame retardant, molded plastic panel fastened to a rugged metal mounting bar to provide sturdiness. The C(G)-310 connector may be used on central offi ce mainframes with 203 mm (8 inch) between verticals. The C(G)-310 connector accepts the full line of industry standard 303-type, 5-pin protector modules including Bourns® hybrid, solid-state and gas tube modules. Specifi cations Plastic Materials Main Body ...........................................................................Polycarbonate, ivory, UL 94V-0 Metal Parts Mounting Hardware .............................................................Steel, -

Digraphs Th, Sh, Ch, Ph
At the Beach Digraphs th, sh, ch, ph • Generalization Words can have two consonants together that are pronounced as one sound: southern, shovel, chapter, hyRJlen. Word Sort Sort the list words by digraphs th, sh, ch, and ph. th ch 1. shovel 2. southern 1. 11. 3. northern 4. chapter 2. 12. 5. hyphen 6. chosen 7. establish 3. 13. 8. although 9. challenge 10. approach 4. 14. 11. astonish 12. python 5. 15. 13. shatter 0 14. ethnic 15. shiver sh 16. Ul 16. pharmacy .,; 6. ..~ ~ 17. charity a: !l 17. .c 18. china CJ) a: 19. attach 7. ~.. 20. ostrich i 18. ~.. 8. "'5 .; £ c ph 0 ~ C) 9. 19. ;ii" c: <G~ ,f 0 E 10. 20. CJ) ·c i;: 0 0 ~ + Home Home Activity Your child is learning about four sounds made with two consonants together, called ~ digraphs. Ask your child to tell you what those four sounds are and give one list word for each sound. DVD•62 Digraphs th, sh, ch, ph Name Unit 2Weel1 1Interactive Review Digraphs th, sh, ch, ph shovel hyphen challenge shatter charity southern chosen approach ethnic china northern establish astonish shiver attach chapter although python pharmacy ostrich Alphabetize Write the ten list words below in alphabetical order. ethnic python ostrich charity hyphen although chapter establish northern southern 1. 6. 2. 7. c, 3. 8. 4. 9. 5. 10. Synonyms Write the list word that has the same or nearly the same meaning. 11. surprise 16. drugstore 12. dare 17. break 13. shake 18. fasten "'u £ c 14. 19. dig 0 pottery ·;; " 15. -

Lexia Lessons Digraph Ch
® LEVEL 6 | Phonics Lexia Lessons Digraph ch Description This lesson is designed to reinforce letter-sound correspondence for the consonant digraph ch, where the two letters, c-h, represent one sound, /ch/. Knowledge of consonant digraphs will improve students’ ability to apply phonic word attack strategies for reading and spelling. TEACHER TIPS The following steps show a lesson in which students identify the sound /ch/ at the beginning of words and match the sound to the letters c-h. For students who have difficulty distinguishing the stop sound /ch/ from the continuant sound /sh/, place emphasis on the stop sound of /ch/ by repeating it while making an abrupt chopping motion with your arm: /ch/ /ch/ /ch/. PREPARATION/MATERIALS • For each student, a card or sticky note • A copy of the ch Keyword Image Card and with the digraph ch printed on it 9 pictures at the end of the lesson • Blank sticky notes Primary Standard: CCSS.ELA-Literacy.RF.1.3a - Know the spelling-sound - Know Standard: CCSS.ELA-Literacy.RF.1.3a Primary correspondences for common consonant digraphs. Warm-up Use a phonemic awareness activity to review how to isolate the initial sound /ch/. ListenasIsayawordintwoparts:/ch/op.Thefirstsoundis/ch/.Theendingsoundsareop.WhenI putthemtogether,Igetthewordchop.Whatisthefirstsoundinchop?(/ch/) Make a single chopping motion with one arm. Thesound/ch/isthesoundwehearatthebeginningofthewordchop.Let’sallsay/ch/together:/ ch//ch//ch/.I’mgoingtosayaword.Ifthewordbeginswith/ch/,chopwithyourhandlikethisand say/ch/.Iftheworddoesnotbeginwith/ch/,keepyourhanddownandmakenosound. Suggested words: chill, chest, time, jump, chain, treasure, gentle, chocolate. Direct Instruction Reading. ® Display the Keyword Image Card of the cheese with the ch on it. -

Ukraine: Country Perspective on C/S Rates
UKRAINE’S EXPERIENCE WITH CAESAREAN SECTIONS: RATES AND INDICATIONS ccording to official statistics the Table 1. Contribution of each C/S indicator to the overall C/S rate in the Donetsk caesarean section (C/S) rate in Region, Ukraine, 2010 and 2012 (aggregated data from 44 maternities). Ukraine increased from 9.2% A Indications for C/S C/S rate by different indications in 1998 to 16.5% in 2012 (1), although according to per total number of deliveries and total C/S this varies across maternities and regions nationally agreed of the country. The Donetsk Region of protocol 2010 (n=41 253) 2012 (n=43 071) Ukraine, with a population of 4.7 million, % of all % of % of all % of has a C/S rate that is higher than the rest N deliveries all C/S N deliveries all C/S of the country. In 2010 there were 41235 deliveries in the Region, with a C/S rate Obstruction for vaginal 486 1.18 6.80 123 0.29 1.61 delivery (pelvic, tissue, of 17.3% and in 2012 there were 43071 tumor) deliveries and the CS rate was 17.7%. Although this C/S rate does not greatly Uterine scar 1559 3.78 21.80 1932 4.49 25.41 exceed the rates recommended by the (previous C/S) WHO of 10-15% (2), we felt that it was Placenta previa/ 595 1.44 8.32 542 1.26 7.13 important to understand the factors as- Placenta abruption sociated with C/S in the Region. Severe preeclampsia 388 0.95 5.43 322 0.75 4.23 Our study had 2 parts. -

C Programming Tutorial
C Programming Tutorial C PROGRAMMING TUTORIAL Simply Easy Learning by tutorialspoint.com tutorialspoint.com i COPYRIGHT & DISCLAIMER NOTICE All the content and graphics on this tutorial are the property of tutorialspoint.com. Any content from tutorialspoint.com or this tutorial may not be redistributed or reproduced in any way, shape, or form without the written permission of tutorialspoint.com. Failure to do so is a violation of copyright laws. This tutorial may contain inaccuracies or errors and tutorialspoint provides no guarantee regarding the accuracy of the site or its contents including this tutorial. If you discover that the tutorialspoint.com site or this tutorial content contains some errors, please contact us at [email protected] ii Table of Contents C Language Overview .............................................................. 1 Facts about C ............................................................................................... 1 Why to use C ? ............................................................................................. 2 C Programs .................................................................................................. 2 C Environment Setup ............................................................... 3 Text Editor ................................................................................................... 3 The C Compiler ............................................................................................ 3 Installation on Unix/Linux ............................................................................ -
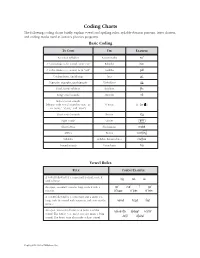
Phonics TRB Coding Chart
Coding Charts The following coding charts briefly explain vowel and spelling rules, syllable-division patterns, letter clusters, and coding marks used in Saxon’s phonics programs. Basic Coding TO CODE USE EXAMPLE Accented syllables Accent marks noÆ C ’s that make a /k/ sound, as in “cat” K-backs |cat C ’s that make a /s/ sound, as in “cell” Cedillas çell Combinations; diphthongs Arcs ar™ Digraphs; trigraphs; quadrigraphs Underlines SH___ Final, stable syllables Brackets [fle Long vowel sounds Macrons nO Schwa vowel sounds (rhymes with vowel sound in “sun,” as Schwas o÷ (or ) in “some,” “about,” and “won”) Short vowel sounds Breves log Sight words Circles ≤are≥ Silent letters Slash marks mak´ Affixes Boxes work ingfl Syllables Syllable division lines cac\tus Voiced sounds Voice lines hiß Vowel Rules RULE CODING EXAMPLE A vowel followed by a consonant is short; code it logcatsit with a breve. An open, accented vowel is long; code it with a nOÆ mEÆ íÆ gOÆ macron. AÆ\|cor™n OÆ\p»n EÆ\v»n A vowel followed by a consonant and a silent e is long; code the vowel with a macron and cross out the nAm´ hOp´ lIk´ silent e. An open, unaccented vowel can make a schwa b«\nanÆ\« E\rAs´Æ hO\telÆ sound. The letters e, o, and u can also make a long sound. The letter i can also make a short sound. JU\lŒÆ di\vId´Æ Copyright by Saxon Publishers, Inc. Spelling Rules† RULE EXAMPLE Floss Rule: When a one-syllable root word has a short vowel sound followed by the sound /f/, /l/, or /s/, it is puff doll pass usually spelled ff, ll, or ss. -

MULTI RAKE SCREEN MRS C-BAR Bar Screen in Stainless Steel
MULTI RAKE SCREEN MRS C-BAR Bar Screen in Stainless Steel Main areas of use and features • Curved bars allow for efficient removal of stones and gravel • Low hydraulic resistance, very low head loss • Bottom step with low blind zone, allows for high hydraulic • Modular delivery possible capacity • Manufacturing as well as installation without welding • High screening discharge capacity due to multiple rakes • Automatic reverese to prevent blocking MRS C-BAR MULTI RAKE SCREEN As an option for difficult installations Meva MRS C-BAR Area of use can be delivered in modules to allow for transportation and assembly of the screen where it otherwise would not be Meva multi-rake bar screen MRS is the optimal choice for possible, later allowing for an easy final assembly on site. installations with difficult operational conditions and/or high screening loads. Reliable function and very low maintenance need of the screen is ensured by the robust design and the non-complicated control. Function In addition, the screen has a high hydraulic capacity, which Meva MRS C-BAR is built with an open structure upstream, makes it possible to operate the screen also with a low head enabling all maintenance and repairs of the drive unit, dead plate loss. or bar rack to be performed without lifting the screen and without access to the back of the screen. The bottom is fitted with long- Meva Multi Rake Screen with curved bars, MRS C-BAR, is a life bearings, for minimum maintenance. modified version of our well-proven MRS. There is no use of brushes or spray water and rake and bars are MRS C-BAR is a mechanically cleaned bar screen suitable individually replaceable.