Change in Serial Laboratory Test Results in Snakebite Patients
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Study-Newcastle-Lonely-Planet.Pdf
Produced by Lonely Planet for Study NT NewcastleDO VIBRAne of Lonely Planet’s Top 10 Cities in Best in Travel 2011 N CREATIVE A LANET Y P ’S EL TO N P O 1 L 0 F TOP C O I T TOP E I E N S O 10 CITY I N 10 CITY ! 1 B 1 E 0 S 2 2011 T L I E N V T A R 2011 PLANE LY T’S NE T O O P L F 1 O 0 C E I N T I O E S ! 1 I 1 N 0 B 2 E L S E T V I A N R T LANET Y P ’S EL TO N P O 1 TOP L 0 F TOP C O I T 10 CITY E I E N S O 10 CITY I N ! 2011 1 B 1 E 0 LAN S P E 2 Y T 2011 T L L ’ I S E N E V T A R N T O O P L F 1 O 0 C E I N T I O E S ! 1 I 1 N 0 B 2 E L S E T V I A N R T E W RE HANI AKBAR st VER I » Age 22 from Saudi Arabia OL » From Saudi Arabia » Studying an International Foundation program What do you think of Newcastle? It’s so beautiful, not big not small, nice. It’s a good place for students who are studying, with a lot of nice people. -

Airds High School Mentoring Program
AIRDS HIGH SCHOOL MENTORING PROGRAM ......................................................................... 21870 ALBURY ELECTORATE AWARD RECIPIENTS ............................................................................ 21866 ALBURY ELECTORATE QUEEN'S BIRTHDAY HONOURS RECIPIENT ................................... 21866 APPROPRIATION (PARLIAMENT) BILL 2013 ................................................................... 21811, 21885 APPROPRIATION BILL 2013 ................................................................................................ 21811, 21885 ARLENE BLENCOWE WORLD BOXING TITLE HOLDER .......................................................... 21865 AUTISM ADVISORY AND SUPPORT SERVICE CHARITY BALL .............................................. 21863 BANKSTOWN LEGACY.................................................................................................................... 21888 BARDEN RIDGE SPORTS COMPLEX ............................................................................................. 21878 BARRENJOEY HIGH SCHOOL BAND ............................................................................................ 21864 BERRY WALKWAY PROJECT ......................................................................................................... 21879 BINA JEWISH EDUCATIONAL ORGANISATION ......................................................................... 21863 BISHOP ANTOINE TARABAY ORDINATION ............................................................................... 21863 BOER -

Hunter Investment Prospectus 2016 the Hunter Region, Nsw Invest in Australia’S Largest Regional Economy
HUNTER INVESTMENT PROSPECTUS 2016 THE HUNTER REGION, NSW INVEST IN AUSTRALIA’S LARGEST REGIONAL ECONOMY Australia’s largest Regional economy - $38.5 billion Connected internationally - airport, seaport, national motorways,rail Skilled and flexible workforce Enviable lifestyle Contact: RDA Hunter Suite 3, 24 Beaumont Street, Hamilton NSW 2303 Phone: +61 2 4940 8355 Email: [email protected] Website: www.rdahunter.org.au AN INITIATIVE OF FEDERAL AND STATE GOVERNMENT WELCOMES CONTENTS Federal and State Government Welcomes 4 FEDERAL GOVERNMENT Australia’s future depends on the strength of our regions and their ability to Introducing the Hunter progress as centres of productivity and innovation, and as vibrant places to live. 7 History and strengths The Hunter Region has great natural endowments, and a community that has shown great skill and adaptability in overcoming challenges, and in reinventing and Economic Strength and Diversification diversifying its economy. RDA Hunter has made a great contribution to these efforts, and 12 the 2016 Hunter Investment Prospectus continues this fine work. The workforce, major industries and services The prospectus sets out a clear blueprint of the Hunter’s future direction as a place to invest, do business, and to live. Infrastructure and Development 42 Major projects, transport, port, airports, utilities, industrial areas and commercial develpoment I commend RDA Hunter for a further excellent contribution to the progress of its region. Education & Training 70 The Hon Warren Truss MP Covering the extensive services available in the Hunter Deputy Prime Minister and Minister for Infrastructure and Regional Development Innovation and Creativity 74 How the Hunter is growing it’s reputation as a centre of innovation and creativity Living in the Hunter 79 STATE GOVERNMENT Community and lifestyle in the Hunter The Hunter is the biggest contributor to the NSW economy outside of Sydney and a jewel in NSW’s rich Business Organisations regional crown. -
Newcastle Relocation Guide
Newcastle Relocation Guide Welcome to Newcastle Newcastle Relocation Guide Contents Welcome to Newcastle ......................................................................................................2 Business in Newcastle ......................................................................................................2 Where to Live? ...................................................................................................................3 Renting.............................................................................................................................3 Buying ..............................................................................................................................3 Department of Fair Trading...............................................................................................3 Electoral Information.........................................................................................................3 Local Council .....................................................................................................................4 Rates...................................................................................................................................4 Council Offices ..................................................................................................................4 Waste Collection................................................................................................................5 Stormwater .........................................................................................................................5 -

Newcastle City Birding Route
NEWCASTLE CITY & LOWER HUNTER ESTUARY parking area under the bridge. A good observation area can be found immediately behind information signs. For several BIRDING ROUTE hours around high tide the lagoon may contain large num- bers of Red-necked Avocet, Bar-tailed and Black-tailed INTRODUCTION: Newcastle is the second largest city in New South Wales. It is densely urbanized and has a diverse heavy Godwit, Curlew Sandpiper, Sharp-tailed Sandpiper and a industry that has occupied a large part of the Hunter Estuary, mostly around the South Arm. However, the greatest concentration few Black-winged Stilt, Gull-billed Tern and Caspian Tern. of migratory shorebirds in NSW roost at Eastern Curlew roost around the lagoon margin, the sand Stockton Sandspit and the Kooragang flats and salt marsh. Diminutive waders such as Red-necked Dykes in the North Arm, only 5km from Stint, Red-capped Plover and Black-fronted Dotterel also the city centre. Thus, the Hunter Estuary use the lagoon mar- is the most important coastal wader gin and salt marsh. habitat in the state and is also a Ramsar Check out the listed site of international importance. A mudflats for foraging variety of seabirds can be seen roosting waders, herons, on the Newcastle City foreshore or flying spoonbills and ibis. offshore and preserved areas of natural Listen for Mangrove vegetation, such as Blackbutt Reserve, G e r y g o n e i n Stockton Sandspit support a diversity of bushbirds in the mangroves on the western suburbs. A Newcastle street di- eastern side of the rectory is essential to follow the routes sandspit. -

Conference Registration CONFERENCE SECRETARIAT DESTINATION the NAC Conference Secretariat Newcastle Is Australia’S 7Th Largest City and One of Its Oldest
Conference Registration CONFERENCE SECRETARIAT DESTINATION The NAC Conference Secretariat Newcastle is Australia’s 7th largest city and one of its oldest. PO Box 180 It has a fast growing reputation as a conference destination as MORISSET NSW 2264 it offers a unique blend of big city facilities and country town Tel: 02 4973 6573 friendliness. It is the capital of the Hunter Region and the most Fax: 02 4973 6609 popular tourist destination outside of Sydney in NSW. E-mail: [email protected] Newcastle is the gateway to the attractions of the region Web: www.thenac.com.au including Hunter Valley Wine Country, Lake Macquarie, VENUE the wilderness of the Upper Hunter or the Shores of Port Stephens, renowned for its dolphin population and whale Noah’s On The Beach watching opportunities. Cnr Shortland Esplanade and Zaara Street Newcastle NSW 2300 Listed in the 2010 Lonely Planet guide as one of the top 10 cities Tel: +61 2 4929 5181 to visit, Newcastle has a flourishing food and wine scene in the Fax: +61 2 4926 5208 CBD where you will find small bars and great coffee. “Today’s new Newcastle is a unique blend of imagination, sophistication Quality Hotel Noah’s On the Beach Newcastle is located and laid-back beach surf culture,” raves the guide. “The city opposite spectacular Newcastle Beach, Newcastle’s most now has the most artists per capita nationwide, and the most renowned surf and swim beach, and in the heart of the galleries.” Newcastle East heritage precinct. Newcastle’s Central Business District, Entertainment venues, Newcastle Harbour For further information about Newcastle please visit www. -
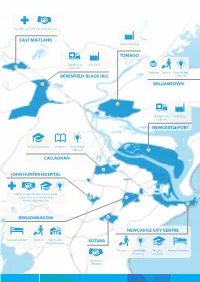
Callaghan East Maitland John Hunter Hospital Kotara
Health Care And Social Assistance EAST MAITLAND Manufacturing TOMAGO Freight and Industrial Logistics Defence Tourism Knowledge BERESFIELD-BLACK HILL Intensive WILLIAMTOWN Freight and Industrial Logistics NEWCASTLE PORT Tertiary Education Research Knowledge intensive CALLAGHAN JOHN HUNTER HOSPITAL Health Care and Social Assistance, Education and Training and Knowledge Intensive BROADMEADOW NEWCASTLE CITY CENTRE Accommodation Tourism Sports and KOTARA Entertainment Tourism Knowledge Tertiary Accommodation Intensive Education Population Serving 2036 CATALYST AREAS FOR GREATER NEWCASTLE NEWCASTLE CITY CENTRE BERESFIELD-BLACK HILL BROADMEADOW CALLAGHAN EAST MAITLAND JOHN HUNTER HOSPITAL KOTARA NEWCASTLE PORT TOMAGO WILLIAMTOWN Draft Greater Newcastle Metropolitan Plan 2036 69 NEWCASTLE CITY CENTRE Transport for NSW will investigate an extension to the ferry network, including a new ferry wharf with pedestrian access to the Newcastle Interchange. Desired role in Greater Newcastle Civic Precinct • Business district, with significant commercial floor space Hunter Development Corporation and Newcastle City Council will: • Metropolitan civic, recreation and cultural facilities, and major events • promote the Civic Precinct as an education and research hub leveraging • Education and innovation precinct from the University of Newcastle NeW Space campus • Urban renewal precinct, meeting demand for medium and high-density • encourage additional civic and cultural housing. activities that reinforce the cultural axis from Civic Park to the waterfront. -

Staff Specialist in Intensive Care Medicine John Hunter Hospital
Staff Specialist in Intensive Care Medicine John Hunter Hospital Applications are invited for multiple permanent and temporary roles as Staff Specialist in Intensive Care Medicine with John Hunter Hospital. We are looking for staff specialists who have demonstrated a collegial, respectful, harmonious and collaborative practice in their previous experience. John Hunter Hospital is a major teaching hospital and is the Primary Tertiary Referral Centre for Trauma in Northern NSW, covering all surgical specialties including Cardiothoracic. Incorporating John Hunter Children’s Hospital, one of the three tertiary Paediatric hospitals in the state. Our busy Intensive Care Unit provides Adult Intensive Care Services and supports a Medical Retrieval Service, also we have a separate Paediatric Intensive Care Unit. ICU inpatient clinical consultation, management and duties may be required to be provided onsite at other public hospitals within HNELHD. Clinical attachment will only be required within Greater Newcastle, including The Maitland Hospital (no greater than 0.5 FTE unless agreed upon). Our ideal candidate: • Will have a strong commitment to multidisciplinary teamwork and quality improvement as well as a strong background in teaching and research. • A primary medical degree registrable with AHPRA and hold Fellowship of the College of Intensive Care Medicine or other specialist recognition as provided for in the Staff Specialist (State) Award or Health Insurance Act (1973) as well as having completed a minimum of 12 months’ work post fellowship. -

Wesley Mission - Green Conscience Wesley Mission - Green Conscience
Wesley Mission - Green Conscience Wesley Mission - Green Conscience Contents Introduction Acknowledgments 1. Birdwood Park 2. Trees in Newcastle 3. Shortland Wetlands 4. Northern Parks & Playgrounds 5. Throsby Creek http://www.wesleymission.org.au/publications/green_c/default.asp (1 of 2) [6/06/2003 3:46:05 PM] Wesley Mission - Green Conscience 6. Hunter Botanic Gardens 1990-2001 7. The Ecohome & Eco-Village 8. Green Point 9. Koala Preservation Society 10. Friends of the Earth 11. Green Corps & Green Reserve 12. Glenrock State Recreation Area 13. Citizens Against Kooragang airport 14. Flora and Fauna Protection Society 15. Smoke Abatement 16. Cleaner beaches 17. Surfrider 18. No Lead Campaign at Boolaroo 19. Australia Native Plant Society 20. Wilderness Society 21. Animal Watch 22. The Green Movement Conclusion Bibliography http://www.wesleymission.org.au/publications/green_c/default.asp (2 of 2) [6/06/2003 3:46:05 PM] Introduction INTRODUCTION We live in a society where conspicuous consumption is often applauded, or envied, rather than deplored. In a society where most of the people live in poverty, the principle that 'more is better' applies. However, when a society becomes affluent this is no longer the case. Many of our problems originate in the fact that some people have not yet grasped this simple truth. One of the problems emanating from this state of affairs is the depletion of natural resources and the pollution of our land, air and water. This book gives a brief account of some of the groups who have attempted to restore a balance, or sanity, into the debate about where we, as a society, are heading. -

Prospects and Challenges for the Hunter Region a Strategic Economic Study
Prospects and challenges for the Hunter region A strategic economic study Regional Development Australia Hunter March 2013 Liability limited by a scheme approved under Professional Standards Legislation. © 2013 Deloitte Access Economics Pty Ltd Prospects and challenges for the Hunter region Contents Executive summary .................................................................................................................... i 1 Introduction .................................................................................................................... 1 Part I: The current and future shape of the Hunter economy .................................................... 3 2 The Hunter economy in 2012 .......................................................................................... 4 2.2 Population and demographics ........................................................................................... 5 2.3 Workforce and employment ............................................................................................. 8 2.4 Industrial composition .................................................................................................... 11 3 Longer term factors and implications ............................................................................ 13 3.1 New patterns in the global economy ............................................................................... 13 3.2 Demographic change ...................................................................................................... 19 -

Lake Macquarie Scenic Management Guidelines 2013 Adopted by Council 11 February 2013 Scenic Management Guidelines
Lake Macquarie Scenic Management Guidelines 2013 Adopted by Council 11 February 2013 Scenic Management Guidelines TABLE OF CONTENTS: PART A: INTRODUCTION .....................................................................................................................9 1 INTRODUCTION ..........................................................................................................................9 1.1 Purpose of this document .........................................................................................................9 1.2 What are scenic and landscape values?...................................................................................9 1.3 Why do we need to protect scenic and landscape values? ......................................................9 1.4 Positive and negative attributes of Lake Macquarie LGA .........................................................9 1.5 Objectives of these guidelines ................................................................................................10 1.6 Methodology............................................................................................................................10 2 HOW TO USE THIS DOCUMENT..............................................................................................12 2.1 General ...................................................................................................................................12 2.2 Council officers - Steps for development assessment or planning proposals.........................12 2.3 -

Team Information Handbook
Commonwealth Fencing Championships Newcastle NSW Australia 19 - 24 November 2002 Team Information Handbook Cf02 Team Information Handbook Page 1 Introduction and Welcome On behalf of the Organisers, the Commonwealth Fencing Federation and the Australian Fencing Federation I would like to welcome all competitors and officials to Newcastle for the Commonwealth Fencing Championship 2002. We hope you enjoy your stay in Newcastle and the tournament and that you achieve the results that you have worked for. When you have done that - we hope that you have FUN! Bill Ronald, OAM Event Director Newcastle The Commonwealth Fencing Championships for 2002 takes place in the harbour and beachside city of Newcastle, Australia's sixth largest city, and the largest non-capital urban area in the nation. With a population of 316,500 people, it is located in the Hunter Region of New South Wales. Venue This tournament will be held in the Newcastle Entertainment Centre with separate areas for pool rounds Direct Elimination phase and finals. This is a major sport and entertainment venue in Newcastle with a seating capacity of 1500 at this tournament. The Centre is a multi-purpose venue, opened in June 1992 has hosted a variety of events, including concerts, circuses, ice shows, basketball, boxing, exhibitions, conventions and now fencing Tourism The Newcastle Pacific Coast region is one of Australia’s top tourist destination. It offers beaches, lakes, national parks, every conceivable sporting opportunity, and is one of Australia's premier winegrowing areas.