KHURSHAID KHAN DEPARTMENT of ZOOLOGY UNIVERSITY of PESHAWAR Session, 2012
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Khyber Pakhtunkhwa - Daily Flood Report Date (29 09 2011)
Khyber Pakhtunkhwa - Daily Flood Report Date (29 09 2011) SWAT RIVER Boundary 14000 Out Flow (Cusecs) 12000 International 10000 8000 1 3 5 Provincial/FATA 6000 2 1 0 8 7 0 4000 7 2 4 0 0 2 0 3 6 2000 5 District/Agency 4 4 Chitral 0 Gilgit-Baltistan )" Gauge Location r ive Swat River l R itra Ch Kabul River Indus River KABUL RIVER 12000 Khyber Pakhtunkhwa Kurram River 10000 Out Flow (Cusecs) Kohistan 8000 Swat 0 Dir Upper Nelam River 0 0 Afghanistan 6000 r 2 0 e 0 v 0 i 1 9 4000 4 6 0 R # 9 9 5 2 2 3 6 a Dam r 3 1 3 7 0 7 3 2000 o 0 0 4 3 7 3 1 1 1 k j n ") $1 0 a Headworks P r e iv Shangla Dir L")ower R t a ¥ Barrage w Battagram S " Man")sehra Lake ") r $1 Amandara e v Palai i R Malakand # r r i e a n Buner iv h J a R n ") i p n Munda n l a u Disputed Areas a r d i S K i K ") K INDUS RIVER $1 h Mardan ia ") ") 100000 li ") Warsak Adezai ") Tarbela Out Flow (Cusecs) ") 80000 ") C")harsada # ") # Map Doc Name: 0 Naguman ") ") Swabi Abbottabad 60000 0 0 Budni ") Haripur iMMAP_PAK_KP Daily Flood Report_v01_29092011 0 0 ") 2 #Ghazi 1 40000 3 Peshawar Kabal River 9 ") r 5 wa 0 0 7 4 7 Kh 6 7 1 6 a 20000 ar Nowshera ") Khanpur r Creation Date: 29-09-2011 6 4 5 4 5 B e Riv AJK ro Projection/Datum: GCS_WGS_1984/ D_WGS_1984 0 Ghazi 2 ") #Ha # Web Resources: http://www.immap.org Isamabad Nominal Scale at A4 paper size: 1:3,500,000 #") FATA r 0 25 50 100 Kilometers Tanda e iv Kohat Kohat Toi R s Hangu u d ") In K ai Map data source(s): tu Riv ") er Punjab Hydrology Irrigation Division Peshawar Gov: KP Kurram Garhi Karak Flood Cell , UNOCHA RIVER $1") Baran " Disclaimers: KURRAM RIVER G a m ") The designations employed and the presentation of b e ¥ Kalabagh 600 Bannu la material on this map do not imply the expression of any R K Out Flow (Cusecs) iv u e r opinion whatsoever on the part of the NDMA, PDMA or r ra m iMMAP concerning the legal status of any country, R ") iv ") e K territory, city or area or of its authorities, or concerning 400 r h ") ia the delimitation of its frontiers or boundaries. -
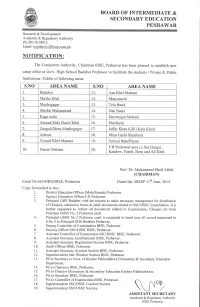
Board Of' Intermediate &
BOARD OF' INTERMEDIATE & SECONDARY EDUCATION PESHAWAR Research & Development/ Academic & Regulatory Authority Ph:091-9218012 Email : [email protected] The Competent Authority, Chairman BISE, Peshawar has been pleased to establish new camp office at Govt. High School Badaber Peshawar to facilitate the students / Private & Public Institutions / Public of followins areas: S.NO AREANAME S.NO AREANAME 1. Badaber 11. Aza Khel Mattani z. Masho Khel 12. Muyamzai 3. Mashogagar lJ. Tela Band A Shiekh Muhammad t4. Nak Band 5. Kaga wala 15. Darwazsai Mattani o" Ahmad Khle Bazid Khel 16. Sherikera 7. ZangalilMer a Masho gagar 17. Jaffar Klan Killi Kala Khel) 8. Adezai 18. Mian Garhi Sherikera o Yousaf Khel Mattani 19. Surizai Bala./Payan F.R Peshawar area i.e Sra Dargai, 10. Pasani Mattani 20. Kandow. Faridi. Bora and Ali Khel. Prof: Dr. Muhammad Shafi Afridi (CHAIRMAN) Endst No.663/DRD,tsISE. Peshawar Dated the. BISEP I 1!' June. 2014 Conv forwarded to the:- l. District Education Officer (Male/Female) Peshawar. 2. Agency Education Officer F.R Peshawar. 3. Principal GHS Badaber with the request to make necessary arrangement for distribution ofCheques, admission forms & other documents related to SSC/HSSC Examination. It is further requested to collect all documents related to Examination, Cheques etc from Principal GHSS No. 2 Peshawar cantt. 4. Principal GHSS No.2 Peshawar cantt is requested to hand over all record mentioned in S.No.3 to Principal GHS Badaber Peshawar. 5. Deputy Controller of Examination BISE, Peshawar. 6. Secrecy Officer SSC/HSSC BISE, Peshawar. 7. Assistant Controller of Examination SSC/HSSC BISE, Peshawar. -

Floristic List and Their Ecological Characteristics, of Plants at Village
Journal of Medicinal Plants Studies 2017; 5(5): 295-299 ISSN (E): 2320-3862 ISSN (P): 2394-0530 Floristic list and their ecological characteristics, NAAS Rating 2017: 3.53 JMPS 2017; 5(5): 295-299 of plants at village Sherpao District Charsadda, © 2017 JMPS Received: 16-07-2017 KP-Pakistan Accepted: 17-08-2017 Sajjad Ali Department of Botany, Bacha Sajjad Ali, Muhammad Shuaib, Hazart Ali, Sami Ullah, Kashif Ali, Khan University Charsadda, Saddam Hussain, Nazim Hassan, Umar Zeb, Wisal Muhammad Khan and Pakistan Fida Hussain Muhammad Shuaib School of Ecology and Environmental Science, Yunnan Abstract University, NO.2 North Cuihu Floral diversity of village Sherpao district Charsadda comprised a total of 104 plant species belonging to road, Kunming, Yunnan, PR. 46 families and 95 genera. The leading families were Fabaceae, Asteraceae and Poaceae contributed by 8 China species each (7.69%) followed by Solanaceae contributed by 7 species (6.73%), while Euphorbiaceae and Hazart Ali Lamiaceae contributed by 5 species each (4.80%) followed by Polygonaceae contributed by 4 species Department of Botany, Bacha (3.84%). Amaranthaceae, Amaryllidaceae, Boraginaceae, Malvaceae, Moraceae, Myrtaceae and Khan University Charsadda, Rutaceae contribute by 3 species each (2.88%) which is followed by Apiaceae, Cannabaceae, Pakistan Caryophyllaceae, Nyctaginaceae, Plantaginaceae, Mimosaceae and Ranunculaceae contributed 2 species Sami Ullah each (1.92%). Rest of 24 families contributed by 1 species each (0.96%). The most dominant life form Department of Botany, University was therophytes having 35 species (33.65%) followed by chamaephytehaving 17 species (16.34%) of Peshawar, Peshawar, Pakistan followed by nanophanerophyte contributed by 15 species. -

Single Bench List for 01-01-2021(Friday)
_ 1 _ PESHAWAR HIGH COURT, PESHAWAR DAILY LIST FOR FRIDAY, 01 JANUARY, 2021 BEFORE:- MR. JUSTICE MUHAMMAD IBRAHIM KHAN Court No: 6 MOTION CASES 1. Cr.M/Q 92- Waqar Shakeel Ahmad Khan Hashtnagri P/2020(model V/s (Charsadda) court (criminal)) State Cr Appeal Branch AG Office 2. CM Corr 405- Muhammad Iqbal Hussain Muhammad Riaz P/2020(in BA V/s 2517-P/2020) State & others Cr Appeal Branch AG Office 3. C.R 783-P/2020 Haji Abid Ullah Liaqat Ali Shinwari With CM No. V/s 1282-P/2020() Hayat Muhammad & others 4. CM 2427/2020 In Bakhtiar Ali Dilawar Khan, Mohib Jan Salarzai, W.P 4185-P/2017 V/s Muhammad Irshad Mohmand (Rent)(stay Noman ud Din alias Mehmood granted on Jan etc Marjan Ali Khan, Muhammad 03/11/2017) Daud Jan 5. CM 2431/2020 In Saif ullah Muhib Kakakhel Saifullah Muhib Kakakhel W.P 7516-P/2019() V/s Federation of Pakistan Asad Jan, Deputy Attorney General, Mian Zia ul Islam, Writ Petition Branch AG Office 6. CM 2447/2020 In Muhammad Tayyab Muhammad Farooq Afridi W.P 5294-P/2020() V/s Collector Customs Deputy Attorney General, Muhammad Jamil IT Branch Peshawar High Court Page 1 of 11 Video Link only available in Court # 1,2,3 and 4 _ 2 _ DAILY LIST FOR FRIDAY, 01 JANUARY, 2021 BEFORE:- MR. JUSTICE MUHAMMAD IBRAHIM KHAN Court No: 6 MOTION CASES 7. I/R In W.P 5719- Nawaz Khan Shakeel Ahmad Khan Hashtnagri P/2020() V/s (Charsadda) Mst Yasmeen Writ Petition Branch AG Office 8. -

Contesting Candidates NA-1 Peshawar-I
Form-V: List of Contesting Candidates NA-1 Peshawar-I Serial No Name of contestng candidate in Address of contesting candidate Symbol Urdu Alphbeticl order Allotted 1 Sahibzada PO Ashrafia Colony, Mohala Afghan Cow Colony, Peshawar Akram Khan 2 H # 3/2, Mohala Raza Shah Shaheed Road, Lantern Bilour House, Peshawar Alhaj Ghulam Ahmad Bilour 3 Shangar PO Bara, Tehsil Bara, Khyber Agency, Kite Presented at Moh. Gul Abad, Bazid Khel, PO Bashir Ahmad Afridi Badh Ber, Distt Peshawar 4 Shaheen Muslim Town, Peshawar Suitcase Pir Abdur Rehman 5 Karim Pura, H # 282-B/20, St 2, Sheikhabad 2, Chiragh Peshawar (Lamp) Jan Alam Khan Paracha 6 H # 1960, Mohala Usman Street Warsak Road, Book Peshawar Haji Shah Nawaz 7 Fazal Haq Baba Yakatoot, PO Chowk Yadgar, H Ladder !"#$%&'() # 1413, Peshawar Hazrat Muhammad alias Babo Maavia 8 Outside Lahore Gate PO Karim Pura, Peshawar BUS *!+,.-/01!234 Khalid Tanveer Rohela Advocate 9 Inside Yakatoot, PO Chowk Yadgar, H # 1371, Key 5 67'8 Peshawar Syed Muhammad Sibtain Taj Agha 10 H # 070, Mohala Afghan Colony, Peshawar Scale 9 Shabir Ahmad Khan 11 Chamkani, Gulbahar Colony 2, Peshawar Umbrella :;< Tariq Saeed 12 Rehman Housing Society, Warsak Road, Fist 8= Kababiyan, Peshawar Amir Syed Monday, April 22, 2013 6:00:18 PM Contesting candidates Page 1 of 176 13 Outside Lahori Gate, Gulbahar Road, H # 245, Tap >?@A= Mohala Sheikh Abad 1, Peshawar Aamir Shehzad Hashmi 14 2 Zaman Park Zaman, Lahore Bat B Imran Khan 15 Shadman Colony # 3, Panal House, PO Warsad Tiger CDE' Road, Peshawar Muhammad Afzal Khan Panyala 16 House # 70/B, Street 2,Gulbahar#1,PO Arrow FGH!I' Gulbahar, Peshawar Muhammad Zulfiqar Afghani 17 Inside Asiya Gate, Moh. -

EASO Country of Origin Information Report Pakistan Security Situation
European Asylum Support Office EASO Country of Origin Information Report Pakistan Security Situation October 2018 SUPPORT IS OUR MISSION European Asylum Support Office EASO Country of Origin Information Report Pakistan Security Situation October 2018 More information on the European Union is available on the Internet (http://europa.eu). ISBN: 978-92-9476-319-8 doi: 10.2847/639900 © European Asylum Support Office 2018 Reproduction is authorised, provided the source is acknowledged, unless otherwise stated. For third-party materials reproduced in this publication, reference is made to the copyrights statements of the respective third parties. Cover photo: FATA Faces FATA Voices, © FATA Reforms, url, CC BY-NC-SA 2.0 Neither EASO nor any person acting on its behalf may be held responsible for the use which may be made of the information contained herein. EASO COI REPORT PAKISTAN: SECURITY SITUATION — 3 Acknowledgements EASO would like to acknowledge the Belgian Center for Documentation and Research (Cedoca) in the Office of the Commissioner General for Refugees and Stateless Persons, as the drafter of this report. Furthermore, the following national asylum and migration departments have contributed by reviewing the report: The Netherlands, Immigration and Naturalization Service, Office for Country Information and Language Analysis Hungary, Office of Immigration and Nationality, Immigration and Asylum Office Documentation Centre Slovakia, Migration Office, Department of Documentation and Foreign Cooperation Sweden, Migration Agency, Lifos -

Administration of Dir Under Nawab Shah Jehan
Pakistan Vol. 49, 2013 Annual Research Journal ADMINISTRATION OF DIR UNDER NAWAB SHAH JEHAN Gohar Ali Shah Abstract Dir the land of lofty mountains, snow peaks, lush green valley, transparent streams and industrious people, remained shrouded for a long time in obscurity, unknown to the outside world. It is very difficult to say that how and when Dir became the residential area but it must be said that due to the beauty, plenty and security, this area will have become the residential area from long age. Dir was invaded by Alexander, than came under the Budhist, the Mughal and important event was the settlement of the Yousafzai tribe in the area by defeating Swatis and Dilazaks in sixteenth century. The followers of Mullah Ilyas ruled for more than three centuries, and then a period of politicization and democratization started. Muhammad Shah Jehan ascended the throne after the death of his father Nawab Aurangzeb khan in November, 1924 and declared himself as the new Nawab of Dir and ruled till 1960. He was a tyrant ruler and ruled with an iron hand. He introduced some administrative reforms in army, judiciary, executive, and in legislation in his principality. His rule was not different than a dictator’s. He snatched the power from his father and imposed his own constitution to show his mighty power. This paper is an attempt to discuss the administrative setup of Nawab Shah Jehan in detail. The data has been collected from books and personal interviews. Keywords Dir, Nawab Shah Jehan, Administration, Jirgah, Shariah, Dastural Amal. Nawab Muhammad Shah Jehan After the death of Nawab Aurangzeb his son Shah Jehan succeeded his father as the Nawab of Dir and ruled from November 1925 to 8th October 1960. -
Khyber Pakhtunkhwa- Peshawar Reference Map (June 14, 2012)
Khyber Pakhtunkhwa- Peshawar Reference Map (June 14, 2012) Legend ! ! ! Settlements M o h m a n d A g e n c y ! ! ! ! ! ! "' Health Facilities WAZIRBAGH Railway Line ! ! ! ! "' ! SHA!GI BAL!A(KHAT!KI) ! ! ! Jogani "' Rivers ! C h a r s a d d a ! C h a r s a d d a ! ! ! ! ! ! Kha! tki ! ! Roads SAEED ABAD ! CHAGHAR MATTI "' FAQIR KILLAYGARA TAJIK"' Motorway ! ! "'! ! "' ! ! ! ! ! ! ! ! Highway HUSSAIN ABAD Gul Bela GUL BELLA ! "' TAKHT ABAD "' "' ! !Gar!hi S! her D!ad ! ! ! ! ! ! ! ! ! ! NASIR BAGH "' "' Primary KAFOOR DHERI Chaghar "'Matti ! "' MATHRA NAHAQI ! "' MATHRA "' KHARAKI Secondary ! Pana!m Dhe!ri ! ! ! ! ! ! ! ! ! ! ! ! ! "' ! ! ! "' CHARPERIZATakhat Abad ! "' Tertiary SUFAID DHERI PUTWAR BALLA KHAZANA ! "' ! ! "'! ! ! ! ! ! ! ! ! ! ! "' Flood Extenct (Oct -Nov 2010) ! ! Nahaqi ! Kaniza Ka!foor D!heri ! ! ! ! ! ! MA! NDRA !KHEL ! ! ! ! ! Peshawar District ! "' DARMANGI K Provincial boundary ! ! ! ! "' ! ! ! Khaza! na ! ! ! ! h "' Haryana Payan ! Mathra PAKHA GHULAM WADPAGA y District boundary ! TARAI PAYAN(SHAQI H.K) "' Kankola "' b ! ! Shahi Bala ! ! ! ! ! ! ! ! ! ! ! e Union Councils PALOSA!IUrban BUDH!AI F A T A "' ! ! "'! r ! Budhni Palosi Pajjagi ! ! P ! ! ! ! ! ! ! ! ! ! ! "' JO!GANI ! JHAGRA a Dag CHAMKAN"'I "' "' ! k REGAI PESHAWAR Laram "'BAZAR KALAN TARNAB FARM k "' "' ! Pakha Ghulam "' RASHID ABAD (NCB) h ! ! ! ! ! ! ! t ISLAMIA COLLEGE HOSPITAL, PESHAWZANANA HOSPITAL, PESHAWAR Wad Paga t ! PHANDOO PAYAN u "' "' "' Regi Palosi Lala n ! Urban Ar! ! ! LANDI ARBAB k Map Doc Name: "' h iMMAP_Peshawar District Reference -

High Casualty Terrorist Bombings (HCTB) Page 1 March 11, 1998 - March 10, 2020
High Casualty Terrorist Bombings (HCTB) Page 1 March 11, 1998 - March 10, 2020 LOC DEATH NINCD MONTH DAY YEAR LOCATION COL 25 3 5 13 1990 Cali, Niza, Quirigua USR 20 1 8 10 1990 Gyandzha, Azerbaijan COL 22 1 2 16 1991 Medellin TUR 36 1 4 9 1991 Istanbul IND 41 2 10 18 1991 Rudrapur ARG 28 1 3 17 1992 Buenos Aires PER 20 1 7 16 1992 Lima PAK 20 1 1 11 1993 Kotri PAK 23 2 1 24 1993 Hyderabad COL 20 1 1 30 1993 Bogota IND 317 13 3 12 1993 Bombay (Mumbai) IND 86 1 3 16 1993 Calcutta COL 15 1 4 15 1993 Bogota IRN 25 1 6 20 1994 Meshed ARG 96 1 7 18 1994 Buenos Aires PAN 21 1 7 19 1994 Colon ISR 23 1 10 19 1994 Tel Aviv ISR 22 2 1 22 1995 Netanya ALG 42 1 1 30 1995 Algiers IRQ 54 1 2 27 1995 Zakho USA 168 1 4 19 1995 Oklahoma City COL 29 1 6 11 1995 Medellin IND 17 1 7 20 1995 Jammu IND 16 1 8 31 1995 Chandigarh SRI 15 2 11 11 1995 Colombo PAK 17 1 11 19 1995 Islamabad IRQ 33 1 11 31 1995 Salahuddin PAK 45 1 12 21 1995 Peshawar SRI 96 1 1 31 1996 Colombo ISR 23 1 2 25 1996 Jerusalem ISR 19 1 3 3 1996 Jerusalem ISR 15 1 3 4 1996 Tel Aviv IND 25 1 3 21 1996 Delhi PAK 60 1 4 28 1996 Bhai Pheri SAU 19 1 6 25 1996 Dhahran SRI 64 1 7 24 1996 Dehiwala TUR 17 1 11 17 1996 Istanbul PER 17 1 12 17 1996 Lima IND 33 1 12 30 1996 Brahmaputra Mail (Assam) CAM 19 4 3 30 1997 Phnom Penh ALG 22 1 4 25 1997 Train near Algiers ALG 15 1 5 3 1997 Sidi Bouhanifa IND 33 1 7 8 1997 Bhatinda ISR 16 2 7 30 1997 Jerusalem SRI 18 1 10 15 1997 Colombo EGY 70 1 11 17 1997 Luxor ALG 103 1 1 11 1998 Sidi Hamed CHN 50 1 2 14 1998 Wuhan IND 46 13 2 14 1998 Coimbatore ALG 18 -
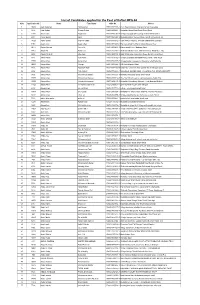
List of Candidates Applied for the Post of Daftari BPS-04 S.No Application No Name Fater Name CNIC No
List of Candidates applied for the Post of Daftari BPS-04 S.No Application No Name Fater Name CNIC No. Addrss 1 15494 Aadil badshah Gul badshah 17101-7677906-1 moh: Dagi mukaram khan p/o tarnab charsadda 2 3214 Aadil Sultan Shuaib Sultan 15602-0930493-7 Mohallah Nasar Khail Saidu Sharif Swat 3 11940 Aamer Jan Sajid khan 17101-7001946-7 Village and post office prang mohalla baba khel te 4 4911 Aamir Sohail Iqbal 15607-0343286-9 Mohallah Chinar Colony,Village and P /O Amankot, M 5 18620 Aamir Sohail Wajeeh Uddin 15607-0365372-7 Swat Plastic Industry, Mohalla Sadbarabad, Balogra 6 1950 Aaqib ullah Aaqib Ullah 15602-9778439-1 Rahman Abad P.o Rahim Abad Mingora Swat 7 10771 Abbas Ahmad Amir Zeb 15607-0339468-9 Rahimabad Tehsel Babozai Swat 8 17711 Abbas Ali Akbar Gul 17301-2133451-1 Shaheen Muslim town, Mohallah Nazer Abad No 2, Hou 9 2403 Abbas Ali Shah Hamayun 15602-9235154-5 Sabir Shah Law Associates Room No.S8,9, 2nd Floor, 10 4185 Abbas Khan Ajab Khan 16101-1106142-7 Village Bughdada Mohallah Mianz Kandi Tehsil & Dis 11 17753 Abbas khan Akbar khan 15302-7569697-7 Village kandaro payee p/o timergara tehsil balamba 12 10963 Abbas Khan Alamgir 15607-0383252-3 PO Box Odigram Swat 13 7518 Abbas Khan Badshah Zada 15602-8847450-9 Falak Naz Jewellers Main Sarafa Bazar Mingora Swat 14 2602 Abbas Khan GUL MALIK 15602-7712428-1 MOHALLA: SAYED ABAD, VILLAGE & P/O: MANGLOR,DISTT: 15 3802 Abbas Khan Muhammad Sardar 15607-0360756-9 Mohalla Afsarabad Saidu Sharif Swat 16 17173 Abbas khan Muhammad Sarwar 15602-0357410-5 The City School near to wali swat -

Valid X-Ray License Holder Sr
Valid X-ray License Holder Sr. Facility Lower Dir 1 Timergara Digital X-ray, Azhar Medical Centre, Near DHQ Hospital, Timergira, Lower Dir 2 Chief X-ray & Clinical Lab., Chakdara, Lower Dir 3 Faisal Digital X-ray, Azhar Plaza, Near DHQ Hospital,Timergira, Lower Dir 4 Anwar X-ray Clinic, Near THQ Hospital, Chakdara, Lower Dir 5 Sadat X-ray, Azher Medical Centre, Near DHQ Hospital, Timergara, Lower Dir 6 Shifa X-ray, Zaib Medical Centre, Near DHQ Hospital, Timergara, Lower Dir 7 New Life Digital X-ray, Zaib Medical Centre, Near DHQ Hospital, Timergara, Lower Dir 8 Sana Clinical lab., Main Road P/O Ouch Tehsil Adenzai, Lower Dir 9 Khairunas Medical Centre, Jnadol Mayar Munda, Lower Dir 10 Hadooko Hospital, Opposite DHQ Hospital, Timergara, Lower Dir 11 Al-Sehat X-ray, Azhar Medical Centre Opssoite DHQ Hospital Timergira, Lower Dir 12 Ali Medical Centre, Village & P.O. Khazana Tehsil Munda, Lower Dir 13 Nasim X-ray, Near THQ Hospital, Samarbagh, Lower Dir 14 PrimeX Digital X-ray, Azhar Plaza, Timergara, Lower Dir 15 Friends Computerized X-ray, Opposite DHQ Hospital, Timergira, Lower Dir 16 Saeed Digital X-ray, Azhar Medical Centre, Near DHQ Hospital, Timergara, Lower Dir 17 Cat-C Hospital, Lal Qila, Lower Dir 18 New Sadaat Digital X-ray, City Medical Centre, Near DHQ Hospital, Timergira, Lower Dir 19 Zam Zam Digital X-ray, City Medical Centre, Timergara, Tehsil Balambat, Lower Dir 20 Madina Medical Centre, Chakdara, Lower Dir 21 City Hospital, Near DHQ Hospital Timergira, Lower Dir Lower Dir Page No. 1 of 1. -

KPK PRISONS DEPARTMENT (KPK-PDHQ) (219)Result for the Post of Male Warder (BPS - 05) Zone 3 PAKISTAN TESTING SERVICE
KPK PRISONS DEPARTMENT (KPK-PDHQ) (219)Result for the post of Male Warder (BPS - 05) Zone 3 PAKISTAN TESTING SERVICE MERIT LIST FOR MALE(ZONE-3) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Higher Total Obtained Marks of Matric Higher Matric Experience Interview Grand Sr # Name Father Name Contact # District Address DOB Height Chest Runnning Qualification Screening Screening Column Division Qualification Marks Marks Marks Total Marks Marks Marks 13+14+17 Ghundo Bala Gul Dherai Po Sakhakot Tehsil 1 Muhammad Asad Khan Yaqoob Khan 03458868881 Malakand April 4, 1993 5x9 37-.39.5 Pass 1st Division Masters and Above 70 12 100 66 148 5 153 Dargai Distt Malaknad 2 Hamid Ullah Aziz Ullah 03458002321 Lower Dir Village Maina Battan Distt Dir Lower Apr 25 1992 5x7 35-36.5 Pass 1st Division Graduation 70 8 100 69 147 4 151 mishta agra p.o totakan teh batkhela distt 3 Azmat hanif seraj ud din 03418441386 Malakand April 8, 1994 5x10 35-38 Pass 1st Division Masters and Above 70 12 100 62 144 4 148 malakand kpk Akram Muran Kalay P.O Koper Tehsil Dengai 4 Muhammad Ismail Juma Din 03438985082 Malakand Mar 14 1989 5x10.5 34-35.5 Pass 1st Division Masters and Above 70 12 100 60 142 4 146 Malakand Village Nari Shah Munda Tehsil Munda Distt 5 Bakhtawar Khan Khair Ur Rahman 03018945393 Lower Dir Apr 8 1992 5x7.5 35.5-37 Pass 1st Division Masters and Above 70 12 100 59 141 5 146 Lower Dir 6 Abdullah Gul Muhammad Khan 03052258845 Lower Dir P O And Vill Ouch Tahsil Adenzai Dist Dir Lower May 8 1994 5x7.5 35.5-36.5 Pass 1st Division Masters and Above 70 12