(HLB) Index Non-Ionic Surfactants on Cyclopentane Hydrates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

+603-4256 6299 / 4256 6376 Fax : +603-4257 3513
Koperasi Ladang Berhad 2-5-14, Prima Peninsula, Jalan Setiawangsa 11, Taman Setiawangsa, 54200 Kuala Lumpur Tel : +603-4256 6299 / 4256 6376 Fax : +603-4257 3513 Email : [email protected] PERAK Bil No.Anggota Nama No. Kad Pengenalan Alamat Terakhir 1 000206 Rohiah Abdullah 0326643 D/A Encik Mohd Abd Kader bin Seeni Aval,Dennis Town Road,Parit Buntar, 2 001074 Wong Sue Ling 0373910 No.33, Kampung Cina,Sitiawan, 3 001080 Ding Ming Kong 0372386 No. 89, Jalan Simpang Dua, Kampung Koh,Sitiawan, 4 001089 Ding Heng Lok 1365557 329, New Village,Kampung Koh,Sitiawan, 5 001174 Phang Lee Hiong 2600134 No.354, Kampung Baru Ayer Tawar,Ayer Tawar, 6 001175 Lau Pek Hua 7737920 No.97,Kampung Baru,Ayer Tawar, 7 001179 Raja Badariah Raja Yusof 1036098 Kampung Kota,Beruas, 8 001181 Rahmah Alang Ibrahim 3841538 Tanjung Belanja,Parit, 9 001185 Ghazali Haji Abdul Wahab 350926-08-5555 1386, Taman Menteri, Changkat Jering,Taiping, 10 001188 Mohd Ramlly Othman 2593724 Sekolah Kebangsaa Kota Lama Kanan,Kuala Kangsar, 11 001191 Haripah Osman 360519-08-5274 Kampung Cheh, Kati,Kuala Kangsar, 12 001194 Zabidah Abd Samad 0376487 98, Jalan Dato' Haron, Kampung Kurnia, Teronoh,Kinta, 13 001197 Mohd Zain Latib 1757984 Suak Blang, Salak Utara,Sungai Siput, 14 001209 Muhammad Amin Hj Ahmad 181216-08-5177 315, Jalan Menteri, Kampung Manjoi,Ipoh, 15 001211 Amjamaliah Alang Mat Yunus 301205-08-5386 Kampung Tanjong, Kampung Kepayang, Sungai Raia,Kinta, 16 001220 Yen Yoon 0377440 B.206, Kampung Pinang, Pusing,Kinta, 17 001223 Chew Chow Khoon 0592835 23, Kampar Road,Ipoh, 18 001240 Duyah Changis 2607242 Kampung Kuala Depang,Kampar, 19 001244 Abd Wahab Yahya 1036887 135, Kampung Tanjung Ara,Parit, 20 001260 Jesah Ngah Maon 2260831 Kampung Periang,Enggor, 21 001266 Suar Hj Zamzam 0587733 d/a Balai Polis, Kg Tawas,Ipoh, 22 001268 Chan Foo Peng 0384435 217, Jalan Chung Ah Ming,Ipoh, 23 001277 Hamsiah binti K.Mat Yunus 331007-08-5120 Kampung Labu Kubong, Lubok Merbau,Kuala Kangsar, 24 001280 Aminah Hassan 0993086 Kampung Baru,Sauk,K. -

Persatuan Geologi Malaysia
KDN 0560/82 ISSN 0126/5539 PERSATUAN GEOLOGI MALAYSIA .NEWSLETTER OF THE GEOLOGICAL SOCIETY OF MALAYSIA Jil. 8, No.5 (Vol. 8, No.5) Sep - Oct 1982 KANDUNGAN (CONTENTS) CATATAN GEOLOGI (GEOLOGICAL NOTES) J.K. Raj: An interesting exposure of unconsolidated sediments in the Kuantan Area of Pahang, Peninsula)" . Malaysia 187 Ibrahim Komoo: Ujian pantulan tukul Schmidt untuk menganggar kekuatan batuan granit di kawasan Pulau Pinang 193 Perbincangan (Discussion) C.S. Hutchison: Occurrence of a not unexpected dolerite in central Kedah - A Discussion 198 T.T. Khoo & B.K. Tan: Occurrence of a not unexpected dolerite in central Kedah - A Reply 199 ! ; PERHUBUNGAN LAIN (OTHER COMMUNICATIONS) i P.H. Stauffer: Alphabetising people's names - it's driving me crazy, Dr. Sigmund! 200 "' T.T. Khoo: Origin of Kinta limestone hills - by George! 202 PERTEMUAN PERSATUAN (MEETINGS OF THE SOCIETY) L. Sandjivy: Geostatistics and tin mining 204 I. Murata: Some aspects of earthquake prediction in Japan 211 J.F. McDivitt: Some aspects of mineral development in the Asean Region 212 GSM Economic Geology Seminar 1982 - Report and Abstracts 217 BERIT A PERSATUAN (NEWS OF THE SOCIETY) GSM Council 1983/84 Election 231 Workshop on Stratigraphic Correlation of Thailand & Malaysia 231 Keahlian (Membership) 234 Keahlian Profesional (Professional Membership) 234 Pertukaran Alamat (Change of Address) 235 Pertambahan Baru Perpustakaan (New Library Additions) 235 BERIT A-BERIT A LAIN (OTHER NEWS) Universiti Malaya Project Reports 1981 /82 236 Seminar on Phosphate Rock Potential -

Jumlah 241 Sekolah Menengah Di Negeri Perak Sepanjang 2012
www.myschoolchildren.com www.myschoolchildren.com BIL NEGERI DAERAH PPD KOD SEKOLAH NAMA SEKOLAH ALAMAT LOKASI BANDAR POSKOD LOKASI NO. TELEFON NO.FAKS L P ENROLMEN 1 PERAK PPD BATANG PADANG AEA0033 SMK HAMID KHAN JALAN DAMAI TAPAH 35000 Bandar 054011181 054014200 369 337 706 2 PERAK PPD BATANG PADANG AEA0034 SMK KHIR JOHARI JALAN BEROP TANJONG MALIM 35900 Bandar 054596334 054582454 391 464 855 3 PERAK PPD BATANG PADANG AEA0035 SMK TROLAK SELATAN FELDA TROLAK SELATAN SUNGKAI 35600 Luar Bandar 054322303 054321125 348 280 628 4 PERAK PPD BATANG PADANG AEA0036 SMK SLIM JLN MELATI, TAMAN SEROJA SLIM RIVER 35800 Luar Bandar 054528926 054520514 359 365 724 5 PERAK PPD BATANG PADANG AEA0037 SMK (FELDA) BESOUT FELDA BESOUT 1 SUNGKAI 35600 Luar Bandar 054311721 054311721 346 360 706 6 PERAK PPD BATANG PADANG AEA0038 SMK SUNGAI KERUIT SIMPANG SUNGAI KLAH SUNGKAI 35600 Luar Bandar 054388308 054388308 230 232 462 7 PERAK PPD BATANG PADANG AEA0039 SMK AIR KUNING JALAN DEGONG MAMBANG DI AWAN 31920 Luar Bandar 054789879 054789879 406 433 839 8 PERAK PPD BATANG PADANG AEA0040 SMK CHENDERIANG KM 2, JALAN TEMOH TAPAH TEMOH 35350 Luar Bandar 054199328 054190145 316 354 670 9 PERAK PPD BATANG PADANG AEA0041 SMK TAPAH JALAN BIDOR LAMA TAPAH 35000 Bandar 054013677 054013677 211 190 401 10 PERAK PPD BATANG PADANG AEA0042 SMK BIDOR JALAN TELUK INTAN BIDOR 35500 Luar Bandar 054347127 054340400 399 411 810 11 PERAK PPD BATANG PADANG AEA0044 SMK BANDAR BEHRANG 2020 BANDAR BEHRANG 2020 TANJONG MALIM 35900 Luar Bandar 054581172 054581168 328 346 674 12 PERAK PPD -

United Nations Code for Trade and Transport Locations (UN/LOCODE) for Malaysia
United Nations Code for Trade and Transport Locations (UN/LOCODE) for Malaysia N.B. To check the official, current database of UN/LOCODEs see: https://www.unece.org/cefact/locode/service/location.html UN/LOCODE Location Name State Functionality Status Coordinatesi MY 2SF Bandar Baru Bangi 10 Road terminal; Recognised location 0257N 10146E MY ABU Abu Port; Request under consideration 0607N 10517E MY AIR Air Itam/Penang 07 Road terminal; Request under consideration 0524N 10017E MY AKA Long Akah 13 Road terminal; Request under consideration 0319N 11447E MY ANG Angsi Port; Request under consideration 0505N 10441E MY AOG Alor Gajah 04 Rail terminal; Road terminal; Recognised location 0222N 10212E MY AOR Alor Setar Airport; Code adopted by IATA or ECLAC MY APG Ampang 10 Road terminal; Recognised location 0309N 10146E MY ASQ Teronoh 08 Port; Multimodal function, ICD etc.; Recognised location 0425N 10059E MY BAG Bagan Datok Port; QQ MY BAH Bahau 05 Multimodal function, ICD etc.; Recognised location 0490N 10225E MY BAI Bangi 10 Road terminal; Recognised location 0254N 10147E MY BAL Balakong 10 Road terminal; Request under consideration 0301N 10145E MY BAN Banting 10 Road terminal; Recognised location 0249N 10129E MY BAT Batu Pahat Port; QQ MY BAU Bau, Sarawak Port; QQ MY BBA Batu Batu, Sabah Port; QQ MY BBE Bukit Beruntung 10 Road terminal; Request under consideration MY BBN Bario Airport; Code adopted by IATA or ECLAC MY BBS Bandar Baru Selayang 10 Multimodal function, ICD etc.; Request under consideration 0316N 10139E MY BCA Batu Caves 10 Road -
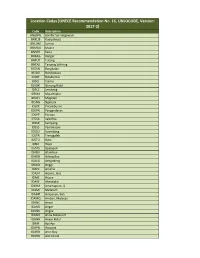
UNECE Recommendation No. 16, UNLOCODE, Version: 2017-2
Location Codes [UNECE Recommendation No. 16, UNLOCODE, Version: 2017-2] Code Description BNBWN Bandar Seri Begawan BNKUB Kuala Belait BNLUM Lumut BNMUA Muara BNSER Seria BNBAG Bangar BNTUT Tutong BNTAS Tanjong Salirong ID5AN Bangkalan ID5BO Bondowoso ID5BT Batubantar ID5CI Ciamis ID5GK Gunung Kidul ID5LE Lembang ID5MA Majalengka ID5MT Magetan ID5NG Nganjuk ID5PE Petamburan ID5PN Pangandaran ID5PP Pacitan ID5SA Salemba ID5SP Sampang ID5SS Pamekasan ID5SU Sumedang ID5TR Trenggalek ID5TU Batu ID6DI Wajo IDAAS Apalapsili IDABU Atambua IDADB Adang Bay IDAEG Aekgodang IDAGD Anggi IDAHI Amahai IDAJN Arjuna, Java IDAJS Arjasa IDAKE Akeselaka IDAMA Amamapare, Ij IDAMI Mataram IDAMP Ampenan, Bali IDAMQ Ambon, Molucas IDANC Ancol IDANG Angar IDANK Angke IDANO Anoa Natuna Pt. IDANR Anyer Kidul IDAPI Api Api IDAPN Ampana IDARB Aroe Bay IDARD Alor Island IDARJ Arso IDASI Asike IDAUN Arun IDAYW Ayawasi IDBA2 Baralaja IDBAD Badau IDBAH Bahudopi IDBAJ Banjarnegara IDBAK Batu Kilat IDBAL Balongan Terminal IDBAM Batuampar IDBAY Banyumas IDBDG Bandengan IDBDJ Banjarmasin IDBDN Buduran IDBDO Bandung, Java IDBEJ Berau IDBEK Bekapai Terminal IDBEN Benete IDBES Bekasi Timur IDBET Belida Terminal IDBGG Banggai IDBIK Biak, Irian Jaya IDBIR Biringkassi IDBIT Bitung, Sulawesi IDBJB Banjarbaru IDBJG Bolaang IDBJI Binjai IDBJN Banjaran IDBJU Banjuwangi, Java IDBJW Bajawa IDBKA Bekasi IDBKI Bengkalis, St IDBKS Bengkulu, Sumatra IDBKY Bengkayang IDBLH Balohan IDBLI Blinju, Banka IDBLL Blang Lancang, St IDBLO Blora IDBLT Belitung IDBLV Beliling IDBLW Belawan, Sumatra -
Single-Solution Simulated Kalman Filter Algorithm for Global Optimisation Problems
Sådhanå (2018) 43:103 Ó Indian Academy of Sciences https://doi.org/10.1007/s12046-018-0888-9Sadhana(0123456789().,-volV)FT3](0123456789().,-volV) Single-solution Simulated Kalman Filter algorithm for global optimisation problems NOR HIDAYATI ABDUL AZIZ1,2,* , ZUWAIRIE IBRAHIM3, NOR AZLINA AB AZIZ1, MOHD SABERI MOHAMAD4 and JUNZO WATADA5 1 Faculty of Engineering and Technology, Multimedia University, 75450 Bukit Beruang, Melaka, Malaysia 2 Faculty of Electrical and Electronics Engineering, Universiti Malaysia Pahang, 26600 Pekan, Pahang, Malaysia 3 Faculty of Manufacturing Engineering, Universiti Malaysia Pahang, 26600 Pekan, Pahang, Malaysia 4 Faculty of Bioengineering and Technology, Universiti Malaysia Kelantan, Jeli Campus, Lock Bag 100, 17600 Jeli, Kelantan, Malaysia 5 Department of Computer and Information Sciences, Universiti Teknologi PETRONAS, Seri Iskandar, 32610 Teronoh, Perak, Malaysia e-mail: [email protected] MS received 26 January 2017; revised 28 September 2017; accepted 6 October 2017; published online 18 June 2018 Abstract. This paper introduces single-solution Simulated Kalman Filter (ssSKF), a new single-agent opti- misation algorithm inspired by Kalman Filter, for solving real-valued numerical optimisation problems. In comparison, the proposed ssSKF algorithm supersedes the original population-based Simulated Kalman Filter (SKF) algorithm by operating with only a single agent, and having less parameters to be tuned. In the proposed ssSKF algorithm, the initialisation parameters are not constants, but they are produced by random numbers taken from a normal distribution in the range of [0, 1], thus excluding them from tuning requirement. In order to balance between the exploration and exploitation in ssSKF, the proposed algorithm uses an adaptive neigh- bourhood mechanism during its prediction step. -

Ipoh Is on the Cusp of Development, but Its Direction Isn't Clear
THE Ipoh is on the cusp of TOWN development, but its direction isn’t clear. Conversations with stakeholders reveal a sense of commitment to retaining the identity of a town that made its fortune and reputation in tin mining, but without a unified THAT vision for the city, how will Ipoh cope with modernisation? Who benefits and who doesn’t? And will the city retain its unique heritage in the face of increasing change? WAS WORDS BY SHERMIAN LIM PHOTOGRAPHS BY KEVIN TEH THAT IS 94 ESQUIRE DECEMBER 2014 ESQUIRE DECEMBER 2014 95 BRILLIANT IDEAS have been conceptualised over Previous spread Any news of development plans that could benefit a better. Critics have voiced frustration over the lack commercial activity there; but without a collective ef- Aerial view of Ipoh in many a pint of lager, and Ian Anderson’s quest to pre- the late ’60s. town is always a welcoming and heartening thing—or of action by the Ipoh City Council to address the fort, unchecked decay has set into parts of the lane. serve Ipoh’s rich historical significance began just is it? issue of buildings that have fallen down, or fol- Meanwhile, private developers continue to Top left like that. The British Royal Navy veteran from Glas- Panglima Lane low through with a well-defined town develop- build high-rise properties on the back of a recent circa 1948: wealthy gow recalls being pictured in a local newspaper 10 tin-mining towkays ment plan that nurtures a local economy. Years housing boom in Ipoh. These properties are tar- years ago, grumbling about the lack of concern over visited their BETWEEN A ROCK AND A TIN PLACE of wasted opportunities, critics claim, have de- geted mainly at overseas investors and have cre- mistresses who Ipoh’s heritage, while having a mug of beer. -

Perak: Senarai Bengkel Penyaman Udara Kenderaan Yang Bertauliah (List of Certified Mobile Air-Conditioning Workshops)
PERAK: SENARAI BENGKEL PENYAMAN UDARA KENDERAAN YANG BERTAULIAH (LIST OF CERTIFIED MOBILE AIR-CONDITIONING WORKSHOPS) NO NAMA SYARIKAT ALAMAT POSKOD DAERAH/BANDAR TELEFON NAMA & K/P COMPANY NAME ADDRESS POST CODE DISTRICT/TOWN TELEPHONE NAME& I/C 1 MEMBAIKI KERETA DAN HAWA NO. 10, JALAN BESAR, 34300 BAGAN SERAI TEL: 05-8905729 CHUA THIEN LI DINGIN SHEN LEE SUNGAI GEDONG, 34300 H/P: 012-4393220 770112-08-6293 BAGAN SERAI, PERAK. 2 PUSAT AKSESORI & HAWA NO. 31, JALAN KELI, TAMAN 34200 BAGAN SERAI TEL: 05-7172208 ENG HUN LIANG DINGIN LIAN HAP. SRI TENGGARA, 34200 PARIT H/P: 012-5677208 830927-08-5701 BUNTAR, PERAK. 3 CHIN AUTO AIRCOND & 523-A, JALAN SIAKAP, BAGAN 34300 BAGAN SERAI TEL : 05-7215807 H'NG SONG CHIN ACCESSORIES SERAI 640906-08-5385 4 WENG HUAT AUTO NO.51, JALAN BESAR, BAGAN 34300 BAGAN SERAI TEL : 05-7215216 KHOH TIANG HUAT ACCESSORIES SERAI 640308-07-5435 5 CHOP BUAN HONG 515, JALAN SIAKAP, BAGAN 34300 BAGAN SERAI TEL : 05-7212734 KHOR HEE SENG 670608- I SERAI 08-6547 6 TEIK SENG CAR ACCESSORIES NO. 182, JALAN SIAKAP, 34300 BAGAN SERAI H/P: 016-4974898 LEE CHENG HOOI AND AIR-COND CENTRE. 34300 BAGAN SERAI, PERAK. 791212-08-6023 1 PERAK: SENARAI BENGKEL PENYAMAN UDARA KENDERAAN YANG BERTAULIAH (LIST OF CERTIFIED MOBILE AIR-CONDITIONING WORKSHOPS) NO NAMA SYARIKAT ALAMAT POSKOD DAERAH/BANDAR TELEFON NAMA & K/P COMPANY NAME ADDRESS POST CODE DISTRICT/TOWN TELEPHONE NAME& I/C 7 BQ CAR ACCESSORIES & AIR NO. 182, JALAN SIAKAP, 34300 BAGAN SERAI TEL : 05-7210812 NG YEOK PHENG COND CENTRE BAGAN SERAI 651225-08-6327 8 K & L ACCESSORIES 73, PRSN. -

Unit Bergerak Spr Perak
PROGRAM OUTREACH BULAN MAC 2011 (PENDAFTARAN DAN SEMAKAN DAFTAR PEMILIH) TARIKH/ BIL. ANJURAN/MASA LOKASI HARI 01/03/11 Program Klinik 1Malaysia 1. Kg. Timah/Changkat Tin, Tanjung Tualang (Selasa) 8.00 pagi – 1.00 petang 01/03/11 Program Klinik 1Malaysia 2. Teronoh Mine, Kampar (Selasa) 2.00 – 5.00 petang 02/03/11 Program Klinik 1Malaysia 3. Jeram, Malim Nawar (Rabu) 8.00 pagi – 1.00 petang 02/03/11 Program Klinik 1Malaysia 4. Kg. Sahom, Malim Nawar (Rabu) 2.00 – 5.00 petang 03/03/11 Program Klinik 1Malaysia 5. Pos Slim, Gunung Rapat (Khamis) 8.00 pagi – 1.00 petang 03/03/11 Program Klinik 1Malaysia 6. Pos Raya, Gunung Rapat (Khamis) 2.00 – 5.00 petang 03/03/11 IPD Hilir Perak 7. Teluk Intan (Khamis) Bermula 8.30 pagi 03/03/11 IPD Sg. Siput 8. Sg. Siput (Khamis) Bermula 8.30 pagi 04/03/11 Program Klinik 1Malaysia 9. Kg. Melayu, Malim Nawar (Jumaat) 8.00 pagi – 1.00 petang 04/03/11 IPD Hilir Perak 10. Teluk Intan (Jumaat) Bermula 8.30 pagi Program Bersama Jabatan Pendaftaran Negara dan Jabatan 12/03/11 12. Kemajuan Orang Asli Sg. Kejar, Gerik (Sabtu) 8.00 pagi – 1.00 petang Program Bersama Jabatan Pendaftaran Negara dan Jabatan 13/03/11 13. Kemajuan Orang Asli Sg. Kejar, Gerik (Ahad) 8.00 pagi – 1.00 petang 16/03/11 Urusan Mendaftarkan Pengundi Bagi Kakitangan Kerajaan 14. Jabatan Pertanian (Rabu) 10.00 pagi 16/03/11 Urusan Mendaftarkan Pengundi Bagi Kakitangan Kerajaan 15. Tenaga Nasional Berhad (Rabu) 10.00 pagi 1 TARIKH/ BIL. -

Negeri Ppd Kod Sekolah Nama Sekolah Alamat Bandar
SENARAI SEKOLAH RENDAH NEGERI PERAK KOD NEGERI PPD NAMA SEKOLAH ALAMAT BANDAR POSKOD TELEFON FAX SEKOLAH PERAK PPD BATANG PADANG ABA0001 SK TOH TANDEWA SAKTI JALAN KELAB TAPAH 35000 054011341 054011341 PERAK PPD BATANG PADANG ABA0002 SK PENDITA ZA'BA JALAN TAPAH ROAD TAPAH ROAD 35400 054182740 054182740 PERAK PPD BATANG PADANG ABA0003 SK BANIR KG. BANIR TAPAH ROAD 35400 054270085 054270085 PERAK PPD BATANG PADANG ABA0004 SK TEMOH KAMPUNG TEMOH STESEN TEMOH 35350 054690253 054690263 PERAK PPD BATANG PADANG ABA0005 SK CHENDERIANG JALAN CHENDERIANG CHENDERIANG 35300 054161461 054161461 PERAK PPD BATANG PADANG ABA0006 SK BIDOR JALAN SUNGKAI BIDOR 35500 054340797 054340797 PERAK PPD BATANG PADANG ABA0007 SK KAMPONG POH KAMPONG POH BIDOR 35500 TIADA TIADA PERAK PPD BATANG PADANG ABA0008 SK BATU TIGA KAMPUNG BATU TIGA TEMOH 35350 054198507 054198507 PERAK PPD BATANG PADANG ABA0009 SK BATU MELINTANG KAMPUNG BATU MELINTANG TAPAH 35000 054270035 054270035 PERAK PPD BATANG PADANG ABA0010 SK HAJI HASAN CHANGKAT PETAI TAPAH ROAD 35400 056591069 056591069 PERAK PPD BATANG PADANG ABA0011 SK SRI KINJANG CHENDERIANG CHENDERIANG 35300 TIADA TIADA PERAK PPD BATANG PADANG ABA0012 SK KAMPONG PAHANG JALAN PAHANG TAPAH 35000 054010396 054010396 PERAK PPD BATANG PADANG ABA0013 SK BATU TUJUH JALAN PAHANG TAPAH 35000 054010735 054010735 PERAK PPD BATANG PADANG ABA0014 SK JERAM MENGKUANG BATU 5, JERAM MENGKUANG BIDOR 35500 054331568 TIADA PERAK PPD BATANG PADANG ABA0015 SK SUNGAI LESONG KG SUNGAI LESONG MAMBANG DI AWAN 31950 054780468 054780468 PERAK PPD BATANG -

Vestigation and Performance Evaluation of Modified Viscoelastic Surfactant (VES) As a New Thickening Fracturing Fluid
polymers Article Experimental Investigation and Performance Evaluation of Modified Viscoelastic Surfactant (VES) as a New Thickening Fracturing Fluid Z. H. Chieng 1, Mysara Eissa Mohyaldinn 1,*, Anas. M. Hassan 1,2 and Hans Bruining 2 1 Department of Petroleum Engineering, Universiti Teknologi PETRONAS (UTP), Seri Iskandar, Teronoh 32610, Perak, Malaysia; [email protected] (Z.H.C.); [email protected] (A.M.H.) 2 Civil Engineering and Geosciences, Delft University of Technology (TU-Delft), Stevinweg 1, 2628 CE Delft, The Netherlands; [email protected] * Correspondence: [email protected] Received: 16 May 2020; Accepted: 25 June 2020; Published: 30 June 2020 Abstract: In hydraulic fracturing, fracturing fluids are used to create fractures in a hydrocarbon reservoir throughout transported proppant into the fractures. The application of many fields proves that conventional fracturing fluid has the disadvantages of residue(s), which causes serious clogging of the reservoir’s formations and, thus, leads to reduce the permeability in these hydrocarbon reservoirs. The development of clean (and cost-effective) fracturing fluid is a main driver of the hydraulic fracturing process. Presently, viscoelastic surfactant (VES)-fluid is one of the most widely used fracturing fluids in the hydraulic fracturing development of unconventional reservoirs, due to its non-residue(s) characteristics. However, conventional single-chain VES-fluid has a low temperature and shear resistance. In this study, two modified VES-fluid are developed as new thickening fracturing fluids, which consist of more single-chain coupled by hydrotropes (i.e., ionic organic salts) through non-covalent interaction. This new development is achieved by the formulation of mixing long chain cationic surfactant cetyltrimethylammonium bromide (CTAB) with organic acids, which are citric acid (CA) and maleic acid (MA) at a molar ratio of (3:1) and (2:1), respectively. -

HUAIKAEO ROAD HUAIKAEO Wat Chet Yod School for Dep.O F Public Woksr There Is No Nationalassembly Bridge
A B c D E Points of Interest for Sightseeing natural rubber. This production supports the economy Wat Sisakat-This temple was built in 1824 by King Points of Interest for Sightseeing LIST OF HOTELS However, you should bear in mind that the roads in of Malaysia. Kuala Lumpur, the capital, was formerly Anouvong. This is one of the most beautiful and Lao PDR are only good in Vientiane proper and a small tin mining town, and developed into the bus Botanic Gardens on Cluny Road- Thousands of exotic tropical pla 8) To prevent the occurrence of the “standing wave” More than 400 roomes Telephone EXIT AND ENTRY PROCEDURES VIENTIANE important temples of the capital with its central KUALA LUMPUR SINGAPORE vicinity, while the roads in the outlying areas are nts. including orchid hybrids, flourish. Boulevard 737-2911 ADVICE TO MOTORISTS phenomenon, the tires should be inflated with 0.3 to tling city it is today. The present population of Kuala Century Pork Sheraton 732-1222 0.5kg/cm2 of air in addition to the regular pressure in poor condition. sanctuary and neat cloisters. Black swans float on a tranquil lake. To reach Vientiane, take the car ferry from Nong- The roads in Malaysia are generally in good con Lumpur is approximately one million. The city has many Dai-Ichi 224-1133 Wat Ong Tu-This temple was built in 1510 by King when driving long distances at high speed. Vientiane is beside the Mekong River and covers a Chinese Gardens (Yu Hwa Yuan)- in Jurong. There are ancient pav Glass 733-0188 khai,Thailand, cross the Mekong River, and get off at Hilton 737-2233 This information is extracted from "Travel Information 1) It is recommended that you contact the Automobile 9) IN CASE OF AN EMERGENCY; The telephone should dition.