From Psoriasis to Psoriatic Arthritis: Insights from Imaging on the Transition to Psoriatic Arthritis and Implications for Arthritis Prevention
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acute Hand Infections
CURRENT CONCEPTS Acute Hand Infections Meredith Osterman, MD, Reid Draeger, MD, Peter Stern, MD CME INFORMATION AND DISCLOSURES The Review Section of JHS will contain at least 3 clinically relevant articles selected by the Provider Information can be found at http://www.assh.org/Pages/ContactUs.aspx. editor to be offered for CME in each issue. For CME credit, the participant must read the Technical Requirements for the Online Examination can be found at http://jhandsurg. articles in print or online and correctly answer all related questions through an online org/cme/home. examination. The questions on the test are designed to make the reader think and will occasionally require the reader to go back and scrutinize the article for details. Privacy Policy can be found at http://www.assh.org/pages/ASSHPrivacyPolicy.aspx. The JHS CME Activity fee of $30.00 includes the exam questions/answers only and does not ASSH Disclosure Policy: As a provider accredited by the ACCME, the ASSH must ensure fi include access to the JHS articles referenced. balance, independence, objectivity, and scienti c rigor in all its activities. Disclosures for this Article Statement of Need: This CME activity was developed by the JHS review section editors Editors and review article authors as a convenient education tool to help increase or affirm fl reader’s knowledge. The overall goal of the activity is for participants to evaluate the Ghazi M. Rayan, MD, has no relevant con icts of interest to disclose. appropriateness of clinical data and apply it to their practice and the provision of patient Authors care. -

Musculoskeletal Ultrasound in the Evaluation of Polymyalgia Rheumatica
Review Med Ultrason 2015, Vol. 17, no. 3, 361-366 DOI: 10.11152/mu.2013.2066.173.aig Musculoskeletal ultrasound in the evaluation of Polymyalgia Rheumatica Iolanda Maria Rutigliano, Chiara Scirocco, Fulvia Ceccarelli, Annacarla Finucci, Annamaria Iagnocco Rheumatology Unit, Sapienza Università di Roma, Rome, Italy Abstract Polymyalgia rheumatica (PMR) is a relatively frequent disease affecting individuals older than 50 years and is character- ized by inflammatory involvement of the shoulder and hip girdles and the neck. Clinical manifestations are represented by pain and morning stiffness in this regions. An extensive and comprehensive assessment of the inflammatory status is crucial in PMR patients, including imaging evaluation. This narrative review reports the current available data in the literature about the role of musculoskeletal ultrasound in PMR. Keywords: polymyalgia rheumatica, ultrasound, bursitis, tenosynovitis Introduction PMR is characterized by pain and morning stiffness, longer than 45 min, involving the neck and the shoulder Polymyalgia rheumatica (PMR) is an inflammatory and hip girdles. Stiffness and pain are usually bilateral, rheumatic condition that typically affects individuals old- worsen in the morning and improve with activity. Fa- er than 50 years, with incidence increasing with age. An tigue, malaise, anorexia, weight loss and fever are also Italian epidemiologic study reported an annual incidence common and are considered “constitutional symptoms”. rate of PMR over the period 1980–1988 of 12.7/100,000 An association between PMR and giant-cell arteritis [1-2]. The etiology of PMR remains unknown, although (GCA) has been described and PMR has been identified currently the role of both genetic and environmental fac- in 40-60% of patients affected by GCA; on the contra- tors, such as infections, has been hypothesized. -

Treatment of the Painful Biceps Tendon—Tenotomy Or Tenodesis?
ARTICLE IN PRESS Current Orthopaedics (2006) 20, 370–375 Available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/cuor UPPER LIMB Treatment of the painful biceps tendon—Tenotomy or tenodesis? F. LamÃ, D. Mok Shoulder Unit, Department of Orthopaedics, Epsom General Hospital, Dorking Road, Epsom, Surrey KT18 7EG, UK KEYWORDS Summary Biceps; The function of the long head of biceps tendon in the shoulder remains controversial. Tenodesis; Pathology of the biceps tendon such as tenosynovitis, subluxation and pre-rupture are Tenotomy; intimately associated with rotator cuff disease. Treatment therefore varies widely among Shoulder surgery surgeons and range from non-operative management to biceps tenotomy or tenodesis. The purpose of this article is to provide an up to date review on the indications and results of biceps tenotomy and tenodesis. & 2006 Elsevier Ltd. All rights reserved. Anatomy Therefore, rupture of the biceps tendon most commonly occurs proximally near the glenoid labrum and distally in the The anatomical origin of the long head of biceps tendon is bicipital groove. variable. It arises most commonly from the glenoid labrum (45%), less commonly from the supraglenoid tubercle (30%) Function and in the remaining it arises from both the glenoid labrum and the supraglenoid tubercle (25%). The tendon travels The biceps muscle-tendon unit is one of many structures in obliquely within the glenohumeral joint to exit beneath the the human body to cross two joints. In the elbow, it serves transverse humeral ligament along the intertubercular primarily as a forearm supinator. Its secondary role is as an sulcus or bicipital groove. In the glenohumeral joint the elbow flexor. -

Giant Bursitis of Wrist and Multiple Tenosynovitis of Hand with Rice Body Formation: Unusual Case of an Atypical Mycobacteria Infection
Open Journal of Rheumatology and Autoimmune Diseases, 2016, 6, 45-50 Published Online August 2016 in SciRes. http://www.scirp.org/journal/ojra http://dx.doi.org/10.4236/ojra.2016.63008 Giant Bursitis of Wrist and Multiple Tenosynovitis of Hand with Rice Body Formation: Unusual Case of an Atypical Mycobacteria Infection Kone Samba1*, Krah K. Leopold2, Traore Moctar3, Gbane Mariam4, Koffi Gerard1, Kouassi Adelaide2, Ngandeu Astrid4 1Traumatology and Orthopedics Surgery of the University Hospital of Cocody, Abidjan, Côte d’Ivoire 2Traumatology and Orthopedics Surgery of the University Hospital of Bouaké, Bouaké, Côte d’Ivoire 3Traumatology and Orthopedics Surgery of the University Hospital of Treichville, Abidjan, Côte d’Ivoire 4Rheumatology Service of the University Hospital of Cocody, Abidjan, Côte d’Ivoire Received 25 March 2016; accepted 6 July 2016; published 9 July 2016 Copyright © 2016 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract We report an unusual manifestation of nontuberculous mycobacterial infection characterized by a giant bursitis on wrist and multiple tenosynovitis with many rice bodies formations. The clinical and radiological examinations are neither rather sensitive nor rather specific. The nuclear im- agery of rice bodies formations provides elements of guidance. Cause of absence of the germ isola- tion, diagnosis was retained on probability items based on a suspicion of arguments beam: clini- cal, biological, bacteriological and histological. The patient was treated with medical and surgical procedure and provided a satisfactory evolution. At follow-up of 15 months, there were no clinical signs of local recurrence. -

Pyogenic Flexor Tenosynovitis of the Hand
5 Points On Pyogenic Flexor Tenosynovitis of the Hand Talia Chapman, MD, and Asif M. Ilyas, MD yogenic flexor tenosynovitis (PFT) is a common closed space infection of the flex- Dr. Chapman is a Resident, Orthopaedic Surgery, Thomas or tendon sheaths of the hand and remains Jefferson University, Philadelphia, Pennsylvania. Dr. Ilyas P is Program Director, Hand and Upper Extremity Surgery, one of the most challenging problems encoun- Rothman Institute, and Associate Professor, Orthopae- tered in orthopedic and hand surgery (Figure 1). dic Surgery, Thomas Jefferson University, Philadelphia, PFT also is known as septic flexor tenosynovitis Pennsylvania. and suppurative flexor tenosynovitis. Authors’ Disclosure Statement: The authors report Kanavel1 initially described 4 cardinal signs no actual or potential conflict of interest in relation that characterize infection of the flexor tendon to this article. sheath: symmetric fusiform swelling of the en- Address correspondence to: Asif M. Ilyas, MD, Rothman tire digit, exquisite tenderness to palpation along Institute, 925 Chestnut St, Philadelphia, PA 19107 the course of the tendon sheath, semiflexed (tel, 610-755-3711; fax, 215-642-3633; email, asif.ilyas@ posture at rest, and pain with attempted passive rothmaninstitute.com). extension of the digit. The prevalence of this Am J Orthop. 2017;46(3):E207-E212. Copyright Frontline infection ranges from 2.5% to 9.4%.2 Once the Medical Communications Inc. 2017. All rights reserved. infection is established in a patient, it can cause significant morbidity and disability and produce an economic burden. It can also present a significant treatment dilemma for the treating managing this common but challenging hand in- surgeon, as there is no standardized protocol for fection. -

De Quervain's Release Standard of Care PT
BRIGHAM & WOMEN’S HOSPITAL Department of Rehabilitation Services Physical Therapy Standard of Care: de Quervain’s Syndrome: Surgical Management Physical Therapy management of the patient who had a release of the first extensor compartment. Case Type/Diagnosis: (diagnosis specific, impairment/dysfunction specific) 11 Since the first description of “washerwoman’s sprain” tenosynovitis of the first dorsal compartment has become a commonly recognized inflammatory disorder. The most radial of the extensor compartments on the dorsum of the wrist is occupied by the tendons of the extensor pollicis brevis and abductor pollicis longus. The tendons are enveloped in an osseofibrous canal lined by synovium, which, when subjected to excessive or repetitive mechanical stresses, responds in a characteristic fashion distinguished by pain, swelling, and limitation of motion of the thumb. In 1895,Fritz de Quervain, a Swiss surgeon, 1 was first credited with the recognition of this disease and so it bore his name. More accurately, Tillaux 2 and Gray 3 referred to this disorder before de Quervain. Anatomy: Twenty-four extrinsic tendons cross the wrist and provide power and dexterity in the hand. Each tendon passes through a series of tight fibrous -osseous canals designed to optimize the balance between motion and force production by maintaining the tendon in close approximation to the joint or joints it controls. There are six separate compartments under the dorsal carpal ligament each lined with a separate synovial sheath membrane. The first one is over the radial styloid and it contains the abductor pollicis longus and the extensor pollicis brevis tendons. These tendons pass through an unyielding osteoligamentous tunnel formed by a shallow groove in the radial styloid process and a tough overlying roof composed by the transverse fibers of the dorsal ligament. -

Imaging Indications in Polymyalgia Rheumatica
REVIEW Imaging indications in polymyalgia rheumatica With the recent improvement in technology, various imaging modalities are increasingly being used in the diagnosis and monitoring of musculoskeletal diseases. Although polymyalgia rheumatica (PMR) is traditionally considered a ‘clinical diagnosis’ the utility of imaging for diagnosis, assessment of disease severity and treatment response in PMR is increasingly recognized. Imaging not only adds to the diagnosis by detecting PMR-specific features, but also helps to make alternative diagnoses. Recently published classification criteria emphasize the importance of ultrasonography, an easily available imaging modality in the diagnosis of PMR. Herein we discuss the role and limitations of ultrasonography, MRI and fludeoxyglucose-PET scanning in the management of PMR, particularly in the diagnosis, and distinguishing it from its numerous mimics. KEYWOD R S: FDG-PET n imaging n MRI n polymyalgia rheumatica n ultrasonograpgy Pravin Patil1, Tochi Adizie1, Shaifali Jain1 The diagnosis of polymyalgia rheumatica (PMR), in the diagnosis of PMR and distinguishing it & Bhaskar Dasgupta*1,2 characterized by proximal pain and stiffness, is from its mimics. In light of the classification 1Southend University Hospital, often uncertain owing to PMR mimics, such as criteria, we also report validation findings from Prittlewell Chase, Westcliff-on-Sea, rheumatoid arthritis (RA), spondyloarthritides, a shoulder ultrasound study of consecutive Essex, SS0 0RY, UK 2Essex University, Colchester, UK inflammatory -
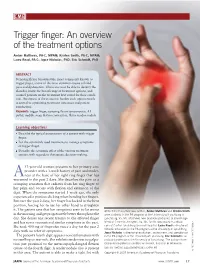
Trigger Finger: an Overview of the Treatment Options
CME Trigger finger: An overview of the treatment options Amber Matthews, PA-C, MPAM; Kristen Smith, PA-C, MPAM; Laura Read, PA-C; Joyce Nicholas, PhD; Eric Schmidt, PhD ABSTRACT Stenosing flexor tenosynovitis, more commonly known as trigger finger, is one of the most common causes of hand pain and dysfunction. Clinicians must be able to identify the disorder, know the broad range of treatment options, and counsel patients on the treatment best suited for their condi- tion. Awareness of the economic burden each option entails is central to optimizing treatment outcomes and patient satisfaction. Keywords: trigger finger, stenosing flexor tenosynovitis, A1 pulley, audible snap, flexion contracture, flexor tendon nodule Learning objectives Describe the typical presentation of a patient with trigger finger. List the commonly used treatments to manage symptoms of trigger finger. Describe the economic effect of the various treatment options with regards to therapeutic decision-making. 53-year-old woman presents to her primary care provider with a 1-week history of pain and tender- Aness at the base of her right ring finger that has worsened in the past 2 days. She describes the pain as a cramping sensation that radiates from her ring finger to her palm and occurs with flexion and extension of the digit. When the symptoms started 1 week ago, she only experienced a painless clicking when bending her fingers. But over the past 2 days, her finger has locked in the bent position, forcing her to use her other hand to straighten it. The patient says that her symptoms seem to be worse At the time this article was written, Amber Matthews and Kristen Smith in the morning and get progressively better throughout the were students in the PA program at the University of Lynchburg in day. -

Enthesopathy of Ankylosing Spondylitis, Are Not Discussed, Nor
394 Book reviews found much of the present knowledge on of their authors to reorientate their International Co-ordination of Drug Trials. the topics covered, including in certain presentations, add other chapters, and Rheumatological Research and the Fight cases the conflicting views. produce the prototype textbook of child- against Rheumatic Disease in Switzerland. Without exception the authors have hood arthritis. Until that time this book Edited by K. Fehr, E. C. Huskisson, and kept to the point and wasted words are should be freely available in every hospital E. Wilhemi. (Pp. 290; illustrated + tables. few. Some of the clinical descriptions are dealing with these diseases. The price is SFr. 32.00, paperback.) Monograph Series, rather formal and stereotyped and the highly competitive by today's standards. No. 1. EULAR Bulletin: Basel. 1977. clinical picture of the disease does not P. J. L. HOLT The 30th Anniversary of the European come across well. Perhaps this is due to League against Rheumatism was cele- the papers being delivered to a peer brated by a meeting in Zurich in April audience rather than for wider con- 1977. The papers and discussion are pub- sumption, the presenters forgetting that lished in the first of a monograph series by the diseases they describe may be un- Peripheral Manipulation. 2nd edition. By EULAR Bulletin through the generosity of familiar to some members of this wider G. D. Maitland. (Pp. 363; illustrated + Merck, Sharp, and Dohme, Zurich. audience. tables. £13.50.) Butterworth: Sevenoaks. Almost two thirds of the monograph is In addition to discussion of the principle 1977. -

Biceps Tendinitis in Chronic Rotator Cuff Tears: a Histologic Perspective
Biceps tendinitis in chronic rotator cuff tears: A histologic perspective Vamsi M. Singaraju, MBBS,a Richard W. Kang, MD,b Adam B. Yanke, MD,b Allison G. McNickle, MS,b Paul B. Lewis, MD,b Vincent M. Wang, PhD,b James M. Williams, PhD,c Susan Chubinskaya, PhD,b,d,e Anthony A. Romeo, MD,b and Brian J. Cole, MD,b,c,f Erie, PA, and Chicago, IL Patients with chronic rotator cuff tears frequently have shoulders than intact constructs.4 Conversely, shoulder anterior shoulder pain attributed to the long head of the function and stabilization are minimally affected in cases of LHBB tendon rupture or surgical re- biceps brachii (LHBB) tendon. In this study, tenodesis or 18,29 tenotomy samples and cadaveric controls were assessed moval. These functional interpretations are not by use of immunohistochemical and histologic methods to without controversy. The role of the LHBB tendon as a source of anterior quantify inflammation, vascularity, and neuronal pain in the shoulder is widely accepted.12,27 This plasticity. Patients had moderate pain and positive results pain is frequently elicited through the palpation of the on at least 1 clinical test of shoulder function. The number anterior shoulder but can also be detected through of axons in the distal LHBB was significantly less in Speed’s20 and Yergason’s33 tests. Chen et al7 showed patients with biceps tendinitis. Calcitonin gene–related significant correlation between rotator cuff tears and bi- peptide and substance P immunostaining was ceps pathology, with all chronic tears (>3 months) hav- predominantly within nerve roots and blood vessels. -

Shoulder Impingement Syndrome
WSCC Clinics Conservative Care Pathways Adopted: 07/98 To be reviewed: 10/99 Reformatted: 02/07 SHOULDER IMPINGEMENT SYNDROME Subacromial Impingement Syndrome Primary Author: Laura L. Baffes, BS, DC, CCSP Co-Author: Lisa Revell, BS, DC Background and Evaluation Sections Contributing Author: Ronald LeFebvre, DC The WSCC Care Pathways provide a standardized context for clinical decision making as well as a menu of possible interventions. These pathways are not intended to replace the clinical judgment of the individual physician. The needs of the individual patient may make it necessary to deviate from the recommendations contained in any given pathway. Limitations WSCC pathways are intended for use within our clinic system. They may be useful as a seed for regional guidelines or guidelines with wider application, but caution must be exercised. The following limitations would have to be addressed. 1) The literature searches employed would need to be more exhaustive; 2) inclusion criteria for published studies would need to be more stringent; 3) a wider pool of subject-matter experts must be tapped; 4) the participants of the consensus panel would need to be drawn from a broader cross-section of the profession and perhaps other health care providers as well. Although individual procedures and decision-making points within the Care Pathways have established validity or reliability, the pathways as a whole are untested. Copyright © 1998 Western States Chiropractic College. Do not reprint without permission. ICD-9 Codes 718.81 Shoulder instability, -

Arthritis, Infectious Tenosynovitis, and Tendon Rupture in a Patient with Rheumatoid Arthritis and Psoriasis
Case in Point Arthritis, Infectious Tenosynovitis, and Tendon Rupture in a Patient With Rheumatoid Arthritis and Psoriasis Peter Vu Bui, MD; and Ifeoma Stella Izuchukwu, MD Multiple comorbidities, advanced age, tobacco use, and alcohol abuse made a proper diagnosis difficult in a patient with polyarticular septic arthritis, infectious tenosynovitis, and ruptures in the tendons of both thumbs. ompared with monoarticular of 31% to 42% compared with 4% to milleri (S. milleri). During surgery, he arthritis, polyarticular arthri- 8% for monoarticular septic arthri- was also found to have bilateral ex- tis may yield an initially nar- tis, and RA was present in 67% of the tensor pollicus longus (EPL) tendon Crower differential diagnosis PASA fatalities.1 rupture. Given the possible morbid- that focuses on systemic inflamma- Rheumatoid arthritis and its treat- ity, the authors believe this patient tory conditions, such as rheumatoid ment predispose patients to septic may be of interest to the medical arthritis (RA). Approximately 15% to arthritis. Septic arthritis in the UK community. 30% of septic arthritis is polyarticu- general population is 0.42 per 100 lar, of which about 45% is associated patient-years for patients with RA on CASE PRESENTATION with underlying RA.1,2 Regardless of antitumor necrosis factor therapy.3,4 A 69-year-old African American male the number of joints involved, septic In a retrospective study in the U.S., presented with 3 to 4 days of swell- (infectious) arthritis is a valid con- the incidence of septic arthritis was ing and pain of bilateral wrists, bi- sideration given the morbidity and 0.40 per 100 patient-years for pa- lateral hands, and the left ankle with mortality.