Determinants of Resource Specialisation and Its Ecological Consequences for the Corallivorous Filefish, Oxymonacanthus Longirostris
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Survey of the Order Tetraodontiformes on Coral Reef Habitats in Southeast Florida
Nova Southeastern University NSUWorks HCNSO Student Capstones HCNSO Student Work 4-28-2020 A Survey of the Order Tetraodontiformes on Coral Reef Habitats in Southeast Florida Anne C. Sevon Nova Southeastern University, [email protected] This document is a product of extensive research conducted at the Nova Southeastern University . For more information on research and degree programs at the NSU , please click here. Follow this and additional works at: https://nsuworks.nova.edu/cnso_stucap Part of the Marine Biology Commons, and the Oceanography and Atmospheric Sciences and Meteorology Commons Share Feedback About This Item NSUWorks Citation Anne C. Sevon. 2020. A Survey of the Order Tetraodontiformes on Coral Reef Habitats in Southeast Florida. Capstone. Nova Southeastern University. Retrieved from NSUWorks, . (350) https://nsuworks.nova.edu/cnso_stucap/350. This Capstone is brought to you by the HCNSO Student Work at NSUWorks. It has been accepted for inclusion in HCNSO Student Capstones by an authorized administrator of NSUWorks. For more information, please contact [email protected]. Capstone of Anne C. Sevon Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science M.S. Marine Environmental Sciences M.S. Coastal Zone Management Nova Southeastern University Halmos College of Natural Sciences and Oceanography April 2020 Approved: Capstone Committee Major Professor: Dr. Kirk Kilfoyle Committee Member: Dr. Bernhard Riegl This capstone is available at NSUWorks: https://nsuworks.nova.edu/cnso_stucap/350 HALMOS -

The Teeth and Dentition of the Filefish (Stephanolepis Cirrhifer) Revisited Tomographically
1 J-STAGE Advance Publication: August 12, 2020 Journal of Oral Science Original article The teeth and dentition of the filefish (Stephanolepis cirrhifer) revisited tomographically Hirofumi Kanazawa1,2), Maki Yuguchi1,2,3), Yosuke Yamazaki1,2,3), and Keitaro Isokawa1,2,3) 1) Division of Oral Structural and Functional Biology, Nihon University Graduate School of Dentistry, Tokyo, Japan 2) Department of Anatomy, Nihon University School of Dentistry, Tokyo, Japan 3) Division of Functional Morphology, Dental Research Center, Nihon University School of Dentistry, Tokyo, Japan (Received October 31, 2019; Accepted November 26, 2019) Abstract: The upper and lower tooth-bearing jaws of the filefish (Stepha- Teeth of Non-Mammalian Vertebrates, Elsevier, 2017). nolepis cirrhifer) were scanned using a micro-CT system in order to With regard to filefish dentition in Japan, an early morphologic and address the existing gaps between the traditional pictures of the morphol- histologic study of Monocanthus cirrhifer and Cantherines modestus ogy and histology. 2D tomograms, reconstructed 3D models and virtual (synonyms Stephanolepis cirrhifer and Thamnaconus modestus, respec- dissection were employed to examine and evaluate the in situ geometry of tively) was carried out by Sohiti Isokawa (Isokawa, Zool Mag 64, 194-197, tooth implantation and the mode of tooth attachment both separately and 1955). Phylogenetic interrelationships in the balistoids were examined collectively. No distinct sockets comparable to those in mammals were extensively by Matsuura [4], based on many anatomical characteristics evident, but shallow depressions were observed in the premaxillary and including the tooth-bearing jaws, which were the premaxillary and the the dentary. The opening of the tooth pulp cavity was not simply oriented dentary. -

First Record of a Filefish, Thamnaconus Tessellatus (Monacanthidae: Tetraodontiformes) from Jeju Island, Korea
KOREAN JOURNAL OF ICHTHYOLOGY, Vol. 29, No. 4, 277-281, December 2017 Received: October 26, 2017 ISSN: 1225-8598 (Print), 2288-3371 (Online) Revised: December 4, 2017 Accepted: December 5, 2017 First Record of a Filefish,Thamnaconus tessellatus (Monacanthidae: Tetraodontiformes) from Jeju Island, Korea By Jeong-Ho Park, Seo Ha Jang, Do Gyun Kim1, Jae-Mook Jeong2, Sukyung Kang and Jin-Koo Kim3,* Fisheries Resource Research Division, National Institute of Fisheries Science (NIFS), Busan 46083, Republic of Korea 1Department of Seafood and Aquaculture Science, Gyeongsang National University, Gyeongnam 53064, Republic of Korea 2Fisheries Resources Research Center, National Institute of Fisheries Science, Gyeongnam 53064, Republic of Korea 3Department of Marine Biology, Pukyong National University, Busan 48513, Republic of Korea ABSTRACT A single specimen (273.1 mm in standard length) of the monacanthid Thamnaconus tessellatus was caught by oneboat trawl from western Jeju Island and then collected at Busan Cooperative Fish Market (BCFM) on 30 May 2015. The specimen is characterized by both head and body with many dark brown spots densely, posterior margin of caudal fin no black, first dorsal spine originates the posterior half of eye, and 34~37 dorsal fin rays. This is the first record of T. tessellatus in Korea; we therefore add the species to the Korean fish fauna. According to the Yamada et al. (1995), we propose the Korean name, “Nambyeoljwichi” for this species. Key words: Monacanthidae, Thamnaconus tessellatus, deep-water leatherjacket, new Korean record, Jeju Island INTRODUCTION is characterized by fully erected first dorsalfin spine (not enveloped in a loose, prominent flap of skin), pelvic fin The family Monacanthidae of the order Tetraodonti rudiment moderate and located at posterior end of pelvis, formes is widely distributed throughout the world, with body depth equal to or greater than length of head length, 107 species in 28 genera (Nelson et al., 2016). -

Ecological Importance of Auxis Spp. As Prey for Dolphin and Wahoo
Ecological importance of Auxis spp. as prey for Dolphin and Wahoo DEPARTMENT OF ENVIRONMENTAL QUALITY Marine Fisheries SAFMC Dolphin/Wahoo Committee| Steve Poland | 12/3/2018 Overview Background • MAFMC request Pelagic Food Web in the SAB • Auxis spp. Important prey in Dolphin/Wahoo diets • Poland thesis – seasonal and size contribution • Rudershausen – annual contribution Questions? 2 MAFMC Unmanaged Forage Omnibus Amendment “To prohibit the development of new and expansion of existing directed commercial fisheries on unmanaged forage species … until the Council has had an adequate opportunity to assess the scientific information relating to any new or expanded directed fisheries and consider potential impacts to existing fisheries, fishing communities, and the marine ecosystem.” Major Actions • Designate taxa included in the amendment as EC species • Manage chub mackerel under discretionary authority • Require EFPs for new fisheries and require comm vessels to be permitted if landing EC species 3 Request to South Atlantic NMFS disapproved measures • Determined inclusion of Auxis spp as a EC species is inconsistent with NS2 • Did not demonstrate the Auxis spp are important forage for MAFMC managed species MAFMC felt that Auxis still warranted protection within its management region • Sent request to SAFMC to consider management of Auxis under its Dolphin/Wahoo FMP Dolphin/Wahoo management unit extends from FL Keys through NY 4 Prey Groups 1. Sargassum associated prey • Filefish, pufferfish, juvenile jacks, swimming crabs 2. Surface schooling prey • Flying fish 3. Schooling prey not assoc. with surface • Bullet tuna, round herring, jacks, cephalopods 4. Small aggregations of crustaceans • Amphipods, stomatopods, isopods Auxis spp. Two species occur in the Atlantic: • A. -

Bleeker, 1856), from Yoron-Jima Island, Ryukyu Islands (Actinopterygii, Tetraodontiformes, Monacanthidae
Bull. Natl. Mus. Nat. Sci., Ser. A, 39(4), pp. 211–213, November 22, 2013 First Record of the Filefish, Pseudomonacanthus macrurus (Bleeker, 1856), from Yoron-jima Island, Ryukyu Islands (Actinopterygii, Tetraodontiformes, Monacanthidae) Keiichi Matsuura1 and Satoru N. Chiba2 1 Department of Zoology, National Museum of Nature and Science, 4–1–1 Amakubo, Tsukuba, Ibaraki, 305–0005 Japan E-mail: [email protected] 2 Center for Molecular Biodiversity Research, National Museum of Nature and Science, 4–1–1 Amakubo, Tsukuba, Ibaraki, 305–0005 Japan E-mail: [email protected] (Received 20 August 2013; accepted 2 October 2013) Abstract A 113-mm SL specimen of a filefish, Pseudomonacanthus macrurus (Bleeker, 1856), was collected with a dip net at a depth of 1 m off the eastern north coast of Yoron-jima Island, Ryukyu Islands. Although juveniles and young specimens were previously recorded from the southern part of Okinawa-jima Island, Ryukyu Islands, the Yoron-jima specimen represents the first record of an adult of P. macrurus from Japan. Key words : Monacanthidae, Tetraodontiformes, distribution, fauna, Japan. vic fin measured from tip of upper lip to poste- Introduction rior end of encasing scales. The specimen is During a survey of the fish fauna of Yoron- deposited at the Kagoshima University Museum jima Island lying in the southern part of Satsunan (KAUM), and a tissue sample of the specimens Group in the Ryukyu Islands, an adult specimen is deposited at the National Museum of Nature of the filefish, Pseudomonacanthus macrurus and Science (NSMT) under the registration num- (Bleeker, 1856), was collected at a depth of 1m ber of NSMT-DNA-60524. -

Intrinsic Vulnerability in the Global Fish Catch
The following appendix accompanies the article Intrinsic vulnerability in the global fish catch William W. L. Cheung1,*, Reg Watson1, Telmo Morato1,2, Tony J. Pitcher1, Daniel Pauly1 1Fisheries Centre, The University of British Columbia, Aquatic Ecosystems Research Laboratory (AERL), 2202 Main Mall, Vancouver, British Columbia V6T 1Z4, Canada 2Departamento de Oceanografia e Pescas, Universidade dos Açores, 9901-862 Horta, Portugal *Email: [email protected] Marine Ecology Progress Series 333:1–12 (2007) Appendix 1. Intrinsic vulnerability index of fish taxa represented in the global catch, based on the Sea Around Us database (www.seaaroundus.org) Taxonomic Intrinsic level Taxon Common name vulnerability Family Pristidae Sawfishes 88 Squatinidae Angel sharks 80 Anarhichadidae Wolffishes 78 Carcharhinidae Requiem sharks 77 Sphyrnidae Hammerhead, bonnethead, scoophead shark 77 Macrouridae Grenadiers or rattails 75 Rajidae Skates 72 Alepocephalidae Slickheads 71 Lophiidae Goosefishes 70 Torpedinidae Electric rays 68 Belonidae Needlefishes 67 Emmelichthyidae Rovers 66 Nototheniidae Cod icefishes 65 Ophidiidae Cusk-eels 65 Trachichthyidae Slimeheads 64 Channichthyidae Crocodile icefishes 63 Myliobatidae Eagle and manta rays 63 Squalidae Dogfish sharks 62 Congridae Conger and garden eels 60 Serranidae Sea basses: groupers and fairy basslets 60 Exocoetidae Flyingfishes 59 Malacanthidae Tilefishes 58 Scorpaenidae Scorpionfishes or rockfishes 58 Polynemidae Threadfins 56 Triakidae Houndsharks 56 Istiophoridae Billfishes 55 Petromyzontidae -

Florida Recreational Saltwater Fishing Regulations
Florida Recreational Issued: July 2020 New regulations are highlighted in red Saltwater Fishing Regulations (please visit: MyFWC.com/Fishing/Saltwater/Recreational Regulations apply to state waters of the Gulf and Atlantic for the most current regulations) All art: © Diane Rome Peebles, except snowy grouper (Duane Raver) Reef Fish Snapper General Snapper Regulations: • Snapper Aggregate Bag Limit - Within state waters ul of the Atlantic and Gulf, Snapper, Cubera u l Snapper, Red u l X Snapper, Vermilion X Snapper, Lane u l all species of snapper are Minimum Size Limits: Minimum Size Limits: Minimum Size Limits: Minimum Size Limits: included in a 10 fish per • Atlantic and Gulf - 12" (see below) • Atlantic - 20" • Atlantic - 12" • Atlantic and Gulf - 8" harvester per day aggregate • Gulf - 16" • Gulf - 10" bag limit in any combination Daily Recreational Bag Limit: Daily Recreational Bag Limit: of snapper species, unless • Atlantic and Gulf - 10 per harvester Season: Daily Recreational Bag Limit: • Atlantic - 10 per harvester stated otherwise. under 30", included within snapper • Atlantic - Open year-round • Atlantic - 5 per harvester not included • Gulf - 100 pounds per harvester, not • Seasons – If no seasonal aggregate bag limit • Gulf - Open June 11–July 25 within snapper aggregate bag limit included within snapper aggregate • May additionally harvest up to 2 over • Gulf - 10 per harvester not included bag limit information is provided, the Daily Recreational Bag Limit: species is open year-round. 30" per harvester or vessel-whichever within snapper aggregate bag limit is less-, and these 2 fish over 30" are • Atlantic and Gulf - 2 per harvester not included within snapper aggregate • Gulf - Zero daily bag and possession limit bag limit for captain and crew on for-hire vessels. -
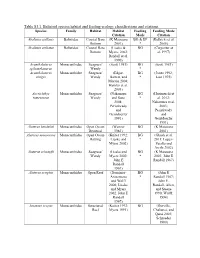
Table S3.1. Balistoid Species Habitat and Feeding Ecology Classifications and Citations
Table S3.1. Balistoid species habitat and feeding ecology classifications and citations. Species Family Habitat Habitat Feeding Feeding Mode Citation Mode Citation Abalistes stellaris Balistidae Coastal Bare (K Matsuura BG & EP (Kulbicki et al. Bottom 2001) * 2005) Abalistes stellatus Balistidae Coastal Bare (Lieske & BG (Carpenter et Bottom Myers, 2002; al. 1997) Randall et al. 1990) Acanthaluteres Monacanthidae Seagrass/ (Scott 1981) BG (Scott 1981) spilomelanurus Weedy * Acanthaluteres Monacanthidae Seagrass/ (Edgar, BG (Jones 1992; vittiger Weedy Barrett, and * Last 1975) Morton 2004; Hyndes et al. 2003) Acreichthys Monacanthidae Seagrass/ (Nakamura BG (Horinouchi et tomentosus Weedy and Sano * al. 2012; 2004; Nakamura et al. Peristiwady 2003; and Peristiwady Geistdoerfer and 1991) Geistdoerfer 1991) Aluterus heudeloti Monacanthidae Open Ocean (Wenner BG (K Matsuura Demersal 1983) 2001) Aluterus monoceros Monacanthidae Open Ocean (Kuiter 1992; BG (Ghosh et al. Rafting Lieske and * 2011; Lopez- Myers 2002) Peralta and Arcila 2002) Aluterus schoepfii Monacanthidae Seagrass/ (Lieske and BG (K Matsuura Weedy Myers 2002; * 2001; John E John E Randall 1967) Randall 1967) Aluterus scriptus Monacanthidae Open Reef (Dominici- BG (John E Arosemena * Randall 1967; and Wolff John E. 2006; Lieske Randall, Allen, and Myers and Steene 2002; John E 1990; Wulff, Randall 1994) 1967) Amanses scopas Monacanthidae Structured (Kuiter 1992; BG (Durville, Reef Myers 1991) Chabanet, and Quod 2003; Schroeder 1980) Balistapus Balistidae Structured (Hiatt and BG & EP (Hiatt and undulatus Reef Strasburg * Strasburg 1960; 1960; Lieske John E. Randall and Myers 1955; John E. 2002; Randall, Allen, McClanahan and Steene and Shafir 1990) 1990; John E. Randall, Allen, and Steene 1990) Balistes capriscus Balistidae Open Ocean (Longley BG & EP (Goldman, Pelagic 1941) * Glasgow, and Falk 2016; Lieske and Myers 2002; Vose and Nelson 1994) Balistes polylepis Balistidae Open Reef (Burgess et BG & EP (Abitia al. -

MONACANTHIDAE Filefishes (Leatherjackets) by K
click for previous page 1970 Bony Fishes MONACANTHIDAE Filefishes (leatherjackets) by K. Matsuura, National Science Museum, Tokyo, Japan iagnostic characters: Small or medium-sized fishes, usually less than 20 cm (but up to 50 cm for some Dspecies of Aluterus), with deep, highly compressed bodies covered by thin but rough or shag- reen-like skin with innumerable minute scales not individually easily discernible to the unaided eye. Mouth small and usually more or less terminal or slightly supraterminal;teeth only moderately heavy,6 in an outer series in upper jaw and 6 or fewer in the lower. Gill opening a relatively short, vertical to oblique slit in front of pectoral-fin base, branchiostegal rays hidden beneath the skin. Two (sometimes 1) dor- sal-fin spines, second spine not more than 1/3 the length of first; first spine usually capable of being locked in an upright position of erection by the second; dorsal-, anal- and pectoral-fin rays unbranched; pelvic fin and spines rudimentary or absent, represented by a series of 3 or fewer pairs of enlarged scales encasing end of pelvis, or segments of indeterminate number, or entirely absent. Scales above pectoral-fin base unmodified, not forming a tympanum. Lateral line inconspicuous or only slightly apparent. Colour: variable, drab brown, grey, or greenish, but often with strikingly marked and vivid patterns. 1st spine prominent 2nd spine fin rays minute unbranched 6 outer teeth 6or fewer teeth restricted gill slit numerous minute encasing scales at scales end of pelvis Habitat, biology,and fisheries: Filefishes range in depth down to about 90 m.They are primarily benthic spe- cies living around coral and rocky reefs or on sand and mud bottoms and seagrass beds. -

Active Reef Fishes Chaetodontidae Chaetodon Fremblii Chaetodon Tinkeri Prognathodes Sp
Marine Fishes Active Reef Fishes Chaetodontidae Chaetodon fremblii Chaetodon tinkeri Prognathodes sp. Pomacanthidae Apolemichthys arcuatus Centropyge fisheri Chaetodon tinkeri Centropyge loricula Courtesy Keoki Stender Genicanthus personatus Pomacentridae Chromis hanui Chromis ovalis Chromis struhsakeri Geniacanthus personatus male Plectroglyphidodon sindonis Courtesy Keoki Stender Priacanthidae Priacanthus meeki Cheilodactylidae Cheilodactylus vittatus Ammodytidae Ammodytoides pylei Lepidammodytes macrophthalamus Monacanthidae Cantherhines verecundus Hawai’i’s State Wildlife Action Plan October 1, 2015 (Last Updated October 2005) Thamnaconus garretti Ostraciidae Ostracion whitleyi Tetraodontidae Torquigener randalli SPECIES STATUS: IUCN Red List – Not considered All Endemic except Chaetodon tinkeri, Apolemichthys, Centropyge loricula, and Ostracion SPECIES INFORMATION: The Hawaiian rock damselfish (Plectroglyphidodon) is territorial. Chaetodon and Centropyge feed on invertebrates and algae. Apolemichthys prefers sponges. Masked Angelfish (Genicanthus) is a protogynous sex changer and primarily feeds on zooplankton and algae. The Chromis species and the ammodytids (sand lances) are also planktivores and often feed in groups. Hawaiian rock damselfish (Plectroglyphidodon) mostly feeds on algae and occasionally on invertebrates. ‘Āweoweo (Priacanthus) is nocturnal and feed on larger zooplankton and may school on occasion. Hawaiian morwong (Cheilodactylus) and Randall’s pufferfish (Torquigener) feed on invertebrates. The monacanthids (filefishes) -

Fish Overview
The Toledo Zoo/ThinkingWorks Teacher Overview for the Fish Lessons Ó2003 Teacher Overview: Fish Fish have many traits that are unique to this particular class of animals. Below is a list of general fish traits to help you and your students complete the ThinkingWorks menu. This lesson focuses on typical fish that most people are familiar with, not on atypical fish such as seahorses. Fish are divided into three groups or classes, each with its own set of features. These classes include the bony fish (e.g., tuna and bass), cartilaginous fish (e.g., sharks and rays) and jawless fish (e.g., lampreys). We have included a list of the different fish found at The Toledo Zoo. Most of the fish are found in the Aquarium but there are also fish in the Diversity of Life. Note that animals move constantly in and out of the Zoo so the list below may be inaccurate. Please call the Zoo for a current list of fish that are on exhibit and their locations. Typical Fish Traits Lightweight, strong scales Lateral line for detecting for protection changes in turbulence along a fish as well as changes in water pressure Gas bladder for buoyancy, stability (internal) Symmetrical tail for Most fish have a well powerful swimming developed eye for locating prey, detecting predators and finding a mate. Flexible “lips” for picking up food Gills for extracting oxygen from the water Maneuverable, paired fins for Lightweight, strong moving forward and controlling skeleton for support roll, pitch and yaw q Fish are cold-blooded, obtaining heat from the surrounding water. -

Salinity Tolerances for the Major Biotic Components Within the Anclote River and Anchorage and Nearby Coastal Waters
Salinity Tolerances for the Major Biotic Components within the Anclote River and Anchorage and Nearby Coastal Waters October 2003 Prepared for: Tampa Bay Water 2535 Landmark Drive, Suite 211 Clearwater, Florida 33761 Prepared by: Janicki Environmental, Inc. 1155 Eden Isle Dr. N.E. St. Petersburg, Florida 33704 For Information Regarding this Document Please Contact Tampa Bay Water - 2535 Landmark Drive - Clearwater, Florida Anclote Salinity Tolerances October 2003 FOREWORD This report was completed under a subcontract to PB Water and funded by Tampa Bay Water. i Anclote Salinity Tolerances October 2003 ACKNOWLEDGEMENTS The comments and direction of Mike Coates, Tampa Bay Water, and Donna Hoke, PB Water, were vital to the completion of this effort. The authors would like to acknowledge the following persons who contributed to this work: Anthony J. Janicki, Raymond Pribble, and Heidi L. Crevison, Janicki Environmental, Inc. ii Anclote Salinity Tolerances October 2003 EXECUTIVE SUMMARY Seawater desalination plays a major role in Tampa Bay Water’s Master Water Plan. At this time, two seawater desalination plants are envisioned. One is currently in operation producing up to 25 MGD near Big Bend on Tampa Bay. A second plant is conceptualized near the mouth of the Anclote River in Pasco County, with a 9 to 25 MGD capacity, and is currently in the design phase. The Tampa Bay Water desalination plant at Big Bend on Tampa Bay utilizes a reverse osmosis process to remove salt from seawater, yielding drinking water. That same process is under consideration for the facilities Tampa Bay Water has under design near the Anclote River.