Seed Dispersal and Dispersal Syndromes in Manzanitas, and Other
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Palm Haven Garden Plant List - 2010
PALM HAVEN GARDEN PLANT LIST - 2010 BOTANICAL NAME COMMON NAME Achillea millefolium 'Paprika' Paprika yarrow Arctostaphylos glauca big berry manzanita Arctostaphylos pajaroensis Pajaro manzanita Arctostaphylos uva-ursi bearberry Arctostaphylos uva-ursi 'San Bruno Mountain' San Bruno Mountain bearberry Aristolochia californica California pipevine Artemisia pycnocephala coastal sagewort Baccharis pilularis 'Twin Peaks' Twin Peaks dwarf coyote brush Berberis aquifolium 'Golden Abundance' Golden Abundance Oregon-grape Carex spissa San Diego sedge Ceanothus 'Joyce Coulter' Joyce Coulte wild lilac Ceanothus arboreus feltleaf ceanothus Ceanothus gloriosus var exaltatus 'Emily Brown' Emily Brown glory brush Dendromecon harfordii Channel Island tree poppy Epilobium canum California fuchsia Erigeron glaucus seaside daisy Eriogonum fasciculatum California buckwheat Eriogonum latifolium coast buckwheat Eriogonum umbellatum sulfur flower Eriophyllum confertiflorum golden yarrow Heteromeles arbutifolia toyon Heterotheca sessilifolora false goldenaster Iris douglasiana Douglas iris Leymus condensatus 'Canyon Prince' Canyon Prince giant wild rye Mimulus aurantiacus sticky monkeyflower Monardella villosa coyote mint Mulhenbergia rigens deer grass Penstemon heterophyllus foothill penstemon Physocarpus capitatus ninebark Rhamnus californica 'Ed Holm' Ed Holm coffeeberry Rhamnus californica 'Mound San Bruno' Mound San Bruno coffeeberry Salvia clevelandii Cleveland sage Salvia mellifera 'Shirley's Creeper' Shirley's Creepe sage Salvia sonomensis 'Bee's Bliss' Bee's Bliss sage Satureja douglasii yerba buena Trichostema lanatum wolly blue curls Typha angustifolia narrow-leaved cattail Verbena lilacina lilac verbena Vitis californica 'Roger's Red' Roger's Red California wild grape. -
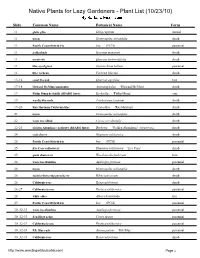
Native Plants for Lazy Gardeners - Plant List (10/23/10)
Native Plants for Lazy Gardeners - Plant List (10/23/10) Slide Common Name Botanical Name Form 11 globe gilia Gilia capitata annual 11 toyon Heteromeles arbutifolia shrub 11 Pacific Coast Hybrid iris Iris (PCH) perennial 11 goldenbush Isocoma menziesii shrub 11 scrub oak Quercus berberidifolia shrub 11 blue-eyed grass Sisyrinchium bellum perennial 11 lilac verbena Verbena lilacina shrub 13-16 coast live oak Quercus agrifolia tree 17-18 Howard McMinn man anita Arctostaphylos 'Howard McMinn' shrub 19 Philip Mun keckiella (RSABG Intro) Keckiella 'Philip Munz' ine 19 woolly bluecurls Trichostema lanatum shrub 19-20 Ray Hartman California lilac Ceanothus 'Ray Hartman' shrub 21 toyon Heteromeles arbutifolia shrub 22 western redbud Cercis occidentalis shrub 22-23 Golden Abundance barberry (RSABG Intro) Berberis 'Golden Abundance' (MAHONIA) shrub 2, coffeeberry Rhamnus californica shrub 25 Pacific Coast Hybrid iris Iris (PCH) perennial 25 Eve Case coffeeberry Rhamnus californica '. e Case' shrub 25 giant chain fern Woodwardia fimbriata fern 26 western columbine Aquilegia formosa perennial 26 toyon Heteromeles arbutifolia shrub 26 fuchsia-flowering gooseberry Ribes speciosum shrub 26 California rose Rosa californica shrub 26-27 California fescue Festuca californica perennial 28 white alder Alnus rhombifolia tree 29 Pacific Coast Hybrid iris Iris (PCH) perennial 30 032-33 western columbine Aquilegia formosa perennial 30 032-33 San Diego sedge Carex spissa perennial 30 032-33 California fescue Festuca californica perennial 30 032-33 Elk Blue rush Juncus patens '.l1 2lue' perennial 30 032-33 California rose Rosa californica shrub http://www weedingwildsuburbia com/ Page 1 30 032-3, toyon Heteromeles arbutifolia shrub 30 032-3, fuchsia-flowering gooseberry Ribes speciosum shrub 30 032-3, Claremont pink-flowering currant (RSA Intro) Ribes sanguineum ar. -

Qty Size Name Price 10 1G Abies Bracteata 12.00 $ 15 1G Abutilon
REGIONAL PARKS BOTANIC GARDEN, TILDEN REGIONAL PARK, BERKELEY, CALIFORNIA Celebrating 78 years of growing California native plants: 1940-2018 **PRELIMINARY**PLANT SALE LIST **PRELIMINARY** Preliminary Plant Sale List 9/29/2018 visit: www.nativeplants.org for the most up to date plant list, updates are posted until 10/5 FALL PLANT SALE OF CALIFORNIA NATIVE PLANTS SATURDAY, October 6, 2018 PUBLIC SALE: 10:00 AM TO 3:00 PM MEMBERS ONLY SALE: 9:00 AM TO 10:00 AM MEMBERSHIPS ARE AVAILABLE AT THE ENTRY TO THE SALE AT 8:30 AM Qty Size Name Price 10 1G Abies bracteata $ 12.00 15 1G Abutilon palmeri $ 11.00 1 1G Acer circinatum $ 10.00 3 5G Acer circinatum $ 40.00 8 1G Acer macrophyllum $ 9.00 10 1G Achillea millefolium 'Calistoga' $ 8.00 25 4" Achillea millefolium 'Island Pink' OUR INTRODUCTION! $ 5.00 28 1G Achillea millefolium 'Island Pink' OUR INTRODUCTION! $ 8.00 6 1G Actea rubra f. neglecta (white fruits) $ 9.00 3 1G Adenostoma fasciculatum $ 10.00 1 4" Adiantum aleuticum $ 10.00 6 1G Adiantum aleuticum $ 13.00 10 4" Adiantum shastense $ 10.00 4 1G Adiantum x tracyi $ 13.00 2 2G Aesculus californica $ 12.00 1 4" Agave shawii var. shawii $ 8.00 1 1G Agave shawii var. shawii $ 15.00 4 1G Allium eurotophilum $ 10.00 3 1G Alnus incana var. tenuifolia $ 8.00 4 1G Amelanchier alnifolia var. semiintegrifolia $ 9.00 8 2" Anemone drummondii var. drummondii $ 4.00 9 1G Anemopsis californica $ 9.00 8 1G Apocynum cannabinum $ 8.00 2 1G Aquilegia eximia $ 8.00 15 4" Aquilegia formosa $ 6.00 11 1G Aquilegia formosa $ 8.00 10 1G Aquilegia formosa 'Nana' $ 8.00 Arabis - see Boechera 5 1G Arctostaphylos auriculata $ 11.00 2 1G Arctostaphylos auriculata - large inflorescences from Black Diamond $ 11.00 1 1G Arctostaphylos bakeri $ 11.00 15 1G Arctostaphylos bakeri 'Louis Edmunds' $ 11.00 2 1G Arctostaphylos canescens subsp. -

Ramirez Dissertation
UC Berkeley UC Berkeley Electronic Theses and Dissertations Title Comparative Ecophysiology and Evolutionary Biology of Island and Mainland Chaparral Communities Permalink https://escholarship.org/uc/item/5b7510px Author Ramirez, Aaron Robert Publication Date 2015 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California Comparative Ecophysiology and Evolutionary Biology of Island and Mainland Chaparral Communities By Aaron Robert Ramirez A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Integrative Biology in the Graduate division of the University of California, Berkeley Committee in charge: Professor David D. Ackerly, Chair Professor Paul V. A. Fine Professor Scott L. Stephens Spring 2015 Comparative Ecophysiology and Evolutionary Biology of Island and Mainland Chaparral Communities © 2015 by Aaron Robert Ramirez Abstract Comparative Ecophysiology and Evolutionary Biology of Island and Mainland Chaparral Communities by Aaron Robert Ramirez Doctor of Philosophy in Integrative Biology University of California, Berkeley Professor David D. Ackerly, Chair The unique nature of island ecosystems have fascinated generations of naturalists, ecologists, and evolutionary biologists. Studying island systems led to the development of keystone biological theories including: Darwin and Wallace’s theories of natural selection, Carlquist’s insights into the biology of adaptive radiations, MacArthur and Wilson’s theory of island biogeography, and many others. Utilizing islands as natural laboratories allows us to discover the underlying fabric of ecology and evolutionary biology. This dissertation represents my attempt to contribute to this long and storied scientific history by thoroughly investigating two aspects of island biology: 1. the role of island climate in shaping drought tolerance of woody plants, and 2. -

Guidelines for Determining Significance and Report Format and Content Requirements
COUNTY OF SAN DIEGO GUIDELINES FOR DETERMINING SIGNIFICANCE AND REPORT FORMAT AND CONTENT REQUIREMENTS BIOLOGICAL RESOURCES LAND USE AND ENVIRONMENT GROUP Department of Planning and Land Use Department of Public Works Fourth Revision September 15, 2010 APPROVAL I hereby certify that these Guidelines for Determining Significance for Biological Resources, Report Format and Content Requirements for Biological Resources, and Report Format and Content Requirements for Resource Management Plans are a part of the County of San Diego, Land Use and Environment Group's Guidelines for Determining Significance and Technical Report Format and Content Requirements and were considered by the Director of Planning and Land Use, in coordination with the Director of Public Works on September 15, 2O1O. ERIC GIBSON Director of Planning and Land Use SNYDER I hereby certify that these Guidelines for Determining Significance for Biological Resources, Report Format and Content Requirements for Biological Resources, and Report Format and Content Requirements for Resource Management Plans are a part of the County of San Diego, Land Use and Environment Group's Guidelines for Determining Significance and Technical Report Format and Content Requirements and have hereby been approved by the Deputy Chief Administrative Officer (DCAO) of the Land Use and Environment Group on the fifteenth day of September, 2010. The Director of Planning and Land Use is authorized to approve revisions to these Guidelines for Determining Significance for Biological Resources and Report Format and Content Requirements for Biological Resources and Resource Management Plans except any revisions to the Guidelines for Determining Significance presented in Section 4.0 must be approved by the Deputy CAO. -

Arctostaphylos Hispidula, Gasquet Manzanita
Conservation Assessment for Gasquet Manzanita (Arctostaphylos hispidula) Within the State of Oregon Photo by Clint Emerson March 2010 U.S.D.A. Forest Service Region 6 and U.S.D.I. Bureau of Land Management Interagency Special Status and Sensitive Species Program Author CLINT EMERSON is a botanist, USDA Forest Service, Rogue River-Siskiyou National Forest, Gold Beach and Powers Ranger District, Gold Beach, OR 97465 TABLE OF CONTENTS Disclaimer 3 Executive Summary 3 List of Tables and Figures 5 I. Introduction 6 A. Goal 6 B. Scope 6 C. Management Status 7 II. Classification and Description 8 A. Nomenclature and Taxonomy 8 B. Species Description 9 C. Regional Differences 9 D. Similar Species 10 III. Biology and Ecology 14 A. Life History and Reproductive Biology 14 B. Range, Distribution, and Abundance 16 C. Population Trends and Demography 19 D. Habitat 21 E. Ecological Considerations 25 IV. Conservation 26 A. Conservation Threats 26 B. Conservation Status 28 C. Known Management Approaches 32 D. Management Considerations 33 V. Research, Inventory, and Monitoring Opportunities 35 Definitions of Terms Used (Glossary) 39 Acknowledgements 41 References 42 Appendix A. Table of Known Sites in Oregon 45 2 Disclaimer This Conservation Assessment was prepared to compile existing published and unpublished information for the rare vascular plant Gasquet manzanita (Arctostaphylos hispidula) as well as include observational field data gathered during the 2008 field season. This Assessment does not represent a management decision by the U.S. Forest Service (Region 6) or Oregon/Washington BLM. Although the best scientific information available was used and subject experts were consulted in preparation of this document, it is expected that new information will arise. -

Cavitation Resistance Among 26 Chaparral Species of Southern California
Ecological Monographs, 77(1), 2007, pp. 99–115 Ó 2007 by the Ecological Society of America CAVITATION RESISTANCE AMONG 26 CHAPARRAL SPECIES OF SOUTHERN CALIFORNIA 1,4 2 1 3 ANNA L. JACOBSEN, R. BRANDON PRATT, FRANK W. EWERS, AND STEPHEN D. DAVIS 1Department of Plant Biology, Michigan State University, East Lansing, Michigan 48824-1312 USA 2Department of Biology, California State University, Bakersfield, 14 SCI, 9001 Stockdale Highway, Bakersfield, California 93311 USA 3Natural Science Division, Pepperdine University, 24255 Pacific Coast Highway, Malibu, California 90263 USA Abstract. Resistance to xylem cavitation depends on the size of xylem pit membrane pores and the strength of vessels to resist collapse or, in the case of freezing-induced cavitation, conduit diameter. Altering these traits may impact plant biomechanics or water transport efficiency. The evergreen sclerophyllous shrub species, collectively referred to as chaparral, which dominate much of the mediterranean-type climate region of southern California, have been shown to display high cavitation resistance (pressure potential at 50% loss of hydraulic conductivity; P50). We examined xylem functional and structural traits associated with more negative P50 in stems of 26 chaparral species. We correlated raw-trait values, without phylogenetic consideration, to examine current relationships between P50 and these xylem traits. Additionally, correlations were examined using phylogenetic independent contrasts (PICs) to determine whether evolutionary changes in these xylem traits correlate with changes in P50. Co-occurring chaparral species widely differ in their P50 (À0.9 to À11.0 MPa). Species experiencing the most negative seasonal pressure potential (Pmin) had the highest resistance to xylem cavitation (lowest P50). Decreased P50 was associated with increased xylem density, stem mechanical strength (modulus of rupture), and transverse fiber wall area when both raw values and PICs were analyzed. -

Qty Size Name 9 1G Abies Bracteata 5 1G Acer Circinatum 4 5G Acer
REGIONAL PARKS BOTANIC GARDEN, TILDEN REGIONAL PARK, BERKELEY, CALIFORNIA Celebrating 77 years of growing California native plants: 1940-2017 **FIRST PRELIMINARY**PLANT SALE LIST **FIRST PRELIMINARY** First Preliminary Plant Sale List 9/29/2017 visit: www.nativeplants.org for the most up to date plant list, updates are posted until 10/6 FALL PLANT SALE OF CALIFORNIA NATIVE PLANTS SATURDAY, October 7, 2017 PUBLIC SALE: 10:00 AM TO 3:00 PM MEMBERS ONLY SALE: 9:00 AM TO 10:00 AM MEMBERSHIPS ARE AVAILABLE AT THE ENTRY TO THE SALE AT 8:30 AM Qty Size Name 9 1G Abies bracteata 5 1G Acer circinatum 4 5G Acer circinatum 7 4" Achillea millefolium 6 1G Achillea millefolium 'Island Pink' 15 4" Achillea millefolium 'Island Pink' 6 1G Actea rubra f. neglecta (white fruits) 15 1G Adiantum aleuticum 30 4" Adiantum capillus-veneris 15 4" Adiantum x tracyi (A. jordanii x A. aleuticum) 5 1G Alnus incana var. tenuifolia 1 1G Alnus rhombifolia 1 1G Ambrosia pumila 13 4" Ambrosia pumila 7 1G Anemopsis californica 6 1G Angelica hendersonii 1 1G Angelica tomentosa 6 1G Apocynum cannabinum 10 1G Aquilegia eximia 11 1G Aquilegia eximia 10 1G Aquilegia formosa 6 1G Aquilegia formosa 1 1G Arctostaphylos andersonii 3 1G Arctostaphylos auriculata 5 1G Arctostaphylos bakeri 10 1G Arctostaphylos bakeri 'Louis Edmunds' 5 1G Arctostaphylos catalinae 1 1G Arctostaphylos columbiana x A. uva-ursi 10 1G Arctostaphylos confertiflora 3 1G Arctostaphylos crustacea subsp. subcordata 3 1G Arctostaphylos cruzensis 1 1G Arctostaphylos densiflora 'James West' 10 1G Arctostaphylos edmundsii 'Big Sur' 2 1G Arctostaphylos edmundsii 'Big Sur' 22 1G Arctostaphylos edmundsii var. -

Drought-Tolerant and Native Plants for Goleta and Santa Barbara County’S Mediterranean Climate
Drought-Tolerant and Native Plants for Goleta and Santa Barbara County’s Mediterranean Climate Drought tolerant plants for the Santa Barbara and Goleta area. In the 1500's California went through an 80 year drought. During the winter there were blizzards in Central California, the Salinas River froze solid where it flowed into the Monterey Bay. During the summer there was no humidity, no rain, and temperatures in the hundreds for many months. During one year in the 1840's there was no measurable rain in Santa Barbara. (The highest measured rainfall in an hour also was in Southern California, 11 inches in an hour) The same native plants that lived through that are still on the hillsides of California. California native plants that do not normally live in the creeks and ponds are very drought tolerant. The best way to find your plant is to check www.mynativeplants.com and do not water at all. But if you want a simple list of drought tolerant plants that can work for your garden here are some. Adenostoma fasciculatum, Chamise. Adenostoma sparsifolium, Red Shanks Agave deserti, Desert Agave Agave shawii, Coastal Agave Agave utahensis, Century Plant Antirrhinum multiflorum, Multiflowered Snapdragon Arctostaphylos La Panza, Grey Manzanita Arctostaphylos densiflora Sentinel Manzanita Arctostaphylos glandulosa adamsii, Laguna Manzanita. Arctostaphylos crustacea eastwoodiana, Harris Grade manzanita. Arctostaphylos glandulosa zacaensis, San Marcos Manzanita Arctostaphylos glauca, Big Berry Manzanita. Arctostaphylos glauca, Ramona Manzanita Arctostaphylos glauca-glandulosa, Weird Manzanita. 1 | Page Arctostaphylos pungens, Mexican Manzanita Arctostaphylos refugioensis Refugio Manzanita Aristida purpurea, Purple 3-awn Artemisia californica, California Sagebrush Artemisia douglasiana, Mugwort Artemisia ludoviciana, White Sagebrush Asclepias fascicularis, Narrowleaf Milkweed Astragalus trichopodus, Southern California Locoweed Atriplex lentiformis Breweri, Brewers Salt Bush. -

Shift of Seed Mass and Fruit Type Spectra Along Longitudinal Gradient: High Water Availability and Growth Allometry
Biogeosciences, 18, 655–667, 2021 https://doi.org/10.5194/bg-18-655-2021 © Author(s) 2021. This work is distributed under the Creative Commons Attribution 4.0 License. Shift of seed mass and fruit type spectra along longitudinal gradient: high water availability and growth allometry Shunli Yu1, Guoxun Wang1, Ofir Katz2, Danfeng Li1, Qibing Wang1, Ming Yue3, and Canran Liu4 1State Key Laboratory of Vegetation and Environmental Change, Institute of Botany, Chinese Academy of Sciences, Beijing, China 2Dead Sea and Arava Science Center, Eilat, Israel 3College of Life Sciences, Northwest University, Xian, China 4Rylah Institute for Environmental Research, Heidelberg, Department of Environment, Land, Water and Planning, Melbourne, VIC 3084, Australia Correspondence: Shunli Yu ([email protected]) Received: 13 December 2019 – Discussion started: 8 June 2020 Revised: 23 November 2020 – Accepted: 8 December 2020 – Published: 28 January 2021 Abstract. Propagule traits vary among biomes along geo- ing leaf area, much more photosynthate (photosynthesis pro- graphical gradients such as longitude, but the mechanisms duction) and allometric growth then ultimately increase the that underlie these variations remain unclear. This study aims biome average seed mass from west to east. Phylogenetic sig- to explore seed mass variation patterns of different biome nal or diversity are not found to be significantly involved in types along a longitudinal gradient and their underlying vari- the effect on the patterns. A novel mechanistic framework ation mechanisms by involving an in-depth analysis on the and mathematical model are provided to expound seed vari- variation of seed mass, fruit type spectra, growth forms and ation among species or biomes. -

Grow Native Nursery Inventory
Grow Native Nursery Inventory As of Dec 21, 2020 Quantity Scientific Name Common Name Size Available Price Acalypha californica California Copperleaf 4 In 3 $ 6.00 Achillea millefolium 'Sonoma Coast' Sonoma Coast Yarrow 1 Gal 10 $ 10.00 Adenostoma fasciculatum Chamise 1 Gal 10 $ 12.00 Agave sebastiana 'Dwarf Form' Small Form Sebastian's Agave 3 Gal 1 $ 45.00 Agave sebastiana 'Dwarf Form' Small Form Sebastian's Agave 4 In 1 $ 28.00 Alnus rhombifolia White Alder 1 Gal 4 $ 12.00 Aloysia wrightii Oreganillo 1 Gal 3 $ 12.00 Aquilegia formosa Western Columbine 4 In 9 $ 6.00 Arctostaphylos 'Austin Griffiths' Austin Griffiths' Manzanita 1 Gal 11 $ 12.00 Arctostaphylos 'Byrd Hill' Byrd Hill's Manzanita 5 Gal 4 $ 45.00 Arctostaphylos 'Dr. Hurd' Dr. Hurd Manzanita 1 Gal 6 $ 12.00 Arctostaphylos edmundsii 'Bert Johnson' Bert Johnson's Manzanita 1 Gal 15 $ 12.00 Arctostaphylos edmundsii 'Carmel Sur' Carmel Sur Manzanita 4 In 23 $ 6.00 Arctostaphylos glauca Bigberry Manzanita 1 Gal 6 $ 15.00 Arctostaphylos 'Howard Mcminn' Howard McMinn's Manzanita 1 Gal 7 $ 12.00 Arctostaphylos 'Howard Mcminn' Howard McMinn's Manzanita 1 Gal 2 $ 12.00 Arctostaphylos 'Lester Rowntree' Lester Rowntree's Manzanita 1 Gal 11 $ 12.00 Arctostaphylos morroensis Morro Bay Manzanita 1 Gal 3 $ 12.00 Arctostaphylos 'Pacific Mist' Pacific Mist Manzanita 1 Gal 29 $ 12.00 Default Arctostaphylos 'Pt. Reyes' Point Reyes Manzanita Title 10 $ 12.00 Arctostaphylos refugioensis Refugio Manzanita 3 Gal 1 $ 26.00 Arctostaphylos refugioensis Refugio Manzanita 1 Gal 15 $ 12.00 Arctostaphylos -

Frugivory and Seed Dispersal in the Endemic Cactus Eulychnia Acida: Extending the Anachronism Hypothesis to the Chilean Mediterranean Ecosystem Rocío A
Cares et al. Revista Chilena de Historia Natural (2018) 91:9 Revista Chilena de https://doi.org/10.1186/s40693-018-0079-4 Historia Natural SHORTREPORT Open Access Frugivory and seed dispersal in the endemic cactus Eulychnia acida: extending the anachronism hypothesis to the Chilean Mediterranean ecosystem Rocío A. Cares1†, Consuelo Sáez-Cordovez1†, Alfonso Valiente-Banuet2, Rodrigo Medel1* and Carezza Botto-Mahan1 Abstract Background: Eulychnia acida is an endemic Chilean cactus species whose fruits show several traits that, taken as a whole, are compatible with a seed dispersal syndrome by large herbivore vertebrates. Since only a few large native mammals exist in Chile at present, cactus fruit consumption and seed dispersal may be coopted by introduced mammals as predicted by Janzen and Martin’s (1982) hypothesis for tropical ecosystems. Findings: We describe the current frugivore species of E. acida in a protected semiarid-Mediterranean ecosystem using field measurements and feeding experiments. In addition, to examine a potential role as seed dispersers of the cactus species, we offered fruits and performed germination tests on seeds defecated by Lama guanicoe and the introduced goat Capra a. hircus under captivity conditions. Our data indicate that while fruits of E. acida are pecked by the Chilean tinamou, Nothoprocta perdicaria, and the Chilean mockingbird, Mimus thenca, and eaten by the brush-tailed rodent, Octodon degus, none of these species could be considered a legitimate seed disperser. Unlike L. guanicoe, the goat C. a. hircus did not reduce seed germination, having a neutral effect. Conclusions: Results from this study indicate that introduced C. a. hircus was the only species showing a potential role in the seed dispersal process of E.