A Thermodynamic Basis for the Electronic Properties of Molten Semiconductors: the Role of Electronic Entropy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

DFT Quantization of the Gibbs Free Energy of a Quantum Body
DFT quantization of the Gibbs Free Energy of a quantum body S. Selenu (Dated: December 2, 2020) In this article it is introduced a theoretical model made in order to perform calculations of the quantum heat of a body that could be acquired or delivered during a thermal transformation of its quantum states. Here the model is mainly targeted to the electronic structure of matter[1] at the nano and micro scale where DFT models have been frequently developed of the total energy of quantum systems. De fining an Entropy functional S[ρ] makes us able optimizing a free energy G[ρ] of the quantum system at finite temperatures. Due to the generality of the model, the latter can be also applied to several first principles computational codes where ab initio modelling of quantum matter is asked. INTRODUCTION thermal transformations at a fi nite temperature T . This work could then be consid- This article will be focused on a study based on the ered as the begining part of a more general theory can derivation of the quantum electrical heat absorbed or ei- include an ab initio modelling of the first law and second ther released by a physical system made of atoms and law of thermodynamics[13], where heat and free energy electrons either at the nano or at the microscopic scale, variations of a quantum body are taken into account. In extending to it the concept of thermal heat within a DFT fact it is actually considered a quantum system of elec- model. Also, the latter being fundamental in physics, can trons at a given temperature T in thermal equilibrium be employed for a full understanding of the thermal phase with its surrounding. -
Resonance As a Measure of Pairing Correlations in the High-Tc
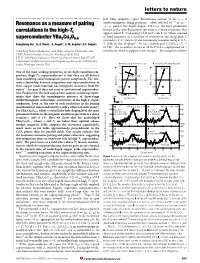
letters to nature ................................................................. lost while magnetic (spin) ¯uctuations centred at the x =0 6±11 Resonance as a measure of pairing antiferromagnetic Bragg positionsÐoften referred to as Q0 = (p, p)±persist. For highly doped (123)O6+x, the most prominent feature in the spin ¯uctuation spectrum is a sharp resonance that correlations in the high-Tc appears below Tc at an energy of 41 meV (refs 6±8). When scanned superconductor YBa Cu O at ®xed frequency as a function of wavevector, the sharp peak is 2 3 6.6 centred at (p, p) (refs 6±8) and its intensity is unaffected by a 11.5- 19 Pengcheng Dai*, H. A. Mook*, G. Aeppli², S. M. Hayden³ & F. DogÏan§ T ®eld in the ab-plane . In our underdoped (123)O6.6 (Tc = 62.7 K)9, the resonance occurs at 34 meV and is superposed on a * Oak Ridge National Laboratory, Oak Ridge, Tennessee 37831-6393, USA continuum which is gapped at low energies11. For frequencies below ² NEC Research Institute, Princeton, New Jersey 08540, USA ³ H. H. Wills Physics Laboratory, University of Bristol, Bristol BS8 1TL, UK § Department of Materials Science and Engineering, University of Washington, Seattle, Washington 98195, USA B rotated 20.6° from the c-axis B along the [3H,-H,0] direction 2.0 2.0 .............................................................................................................................................. a d [H,3H,0] One of the most striking properties of the high-transition-tem- 1.5 1.5 perature (high-Tc) superconductors is that they are all derived [H, (5-H)/3, 0] from insulating antiferromagnetic parent compounds. The inti- 1.0 B ∼ i c-axis 1.0 B i ab-plane mate relationship between magnetism and superconductivity in these copper oxide materials has intrigued researchers from the [0,K,0] (r.l.u.) [0,K,0] (r.l.u.) 0.5 0.5 outset1±4, because it does not exist in conventional superconduc- 20.6° tors. -

Feas: Heat Capacity, Enthalpy Increments, Other Thermodynamic Properties from 5 to 1350 K, and Magnetic Transition A
M-2341 J. Chem. Thermodynumics 1989,21, 363-373 FeAs: Heat capacity, enthalpy increments, other thermodynamic properties from 5 to 1350 K, and magnetic transition a DOMINGO GONZALEZ-ALVAREZ, Fact&ad de Ciencias de la Universidad de Zaragoza, Departamento de Fisica Fundamental, 5009 Zaragoza, Spain FREDRIK GR0NVOLD, Chemical Institute, University of Oslo, Blindern, Oslo 3, Norway BENGT FALK,b EDGAR F. WESTRUM, JR., Department of Chemistry, University of Michigan, Ann Arbor, MI 48109, U.S.A. R. BLACHNIK,’ and G. KUDERMANNd Technical University, Siegen, Federal Republic of Germany (Received 2 January 1989) The heat capacity of iron monoarsenide has been determined by adiabatic calorimetry from 5 to 1030 K and bv drou calorimetrv relative to 298.15 K over the range 875 to 1350 K. A small h-type transition is observed at ?;, = (70.95 kO.02) K. It is related & the disappearance of a doubly helically ordered magnetic-spin structure on heating. The obviously cooperative entropy increment of transition is only A&JR = 0.021. The higher-temperature heat capacity rises considerably above lattice expectations. Part of the rise is ascribed to low-spin electron redistribution in iron, while the further excess above 800 K presumably arises from a beginning low- to high-spin transition, possibly connected with interstitial defect formation in the MnP- type structure. FeAs melts at about 1325 K with A,,,Hk=6180R.K. Thermodynamic functions have been evaluated and the values of C,,,(T), S:(T), H:(T), and @i(T), are 6.057R, 7.513R,1177R.K, and 3.567Rat 298.15 K, and 8.75R,16.03R, 6287R.K, and 9.74512at 1000 K. -

High Temperature Enthalpies of the Lead Halides
HIGH TEMPERATURE ENTHALPIES OF THE LEAD HALIDES: ENTHALPIES AND ENTROPIES OF FUSION APPROVED: Graduate Committee: Major Professor Committee Member Min fessor Committee Member X Committee Member UJ. Committee Member Committee Member Chairman of the Department of Chemistry Dean or the Graduate School HIGH TEMPERATURE ENTHALPIES OF THE LEAD HALIDES: ENTHALPIES AND ENTROPIES OF FUSION DISSERTATION Presented to the Graduate Council of the North Texas State University in Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY By Clarence W, Linsey, B. S., M. S, Denton, Texas June, 1970 ACKNOWLEDGEMENT This dissertation is based on research conducted at the Oak Ridge National Laboratory, Oak Ridge, Tennessee, which is operated by the Uniofl Carbide Corporation for the U. S. Atomic Energy Commission. The author is also indebted to the Oak Ridge Associated Universities for the Oak Ridge Graduate Fellowship which he held during the laboratory phase. 11 TABLE OF CONTENTS Page LIST OF TABLES iv LIST OF ILLUSTRATIONS v Chapter I. INTRODUCTION . 1 Lead Fluoride Lead Chloride Lead Bromide Lead Iodide II. EXPERIMENTAL 11 Reagents Fusion-Filtration Method Encapsulation of Samples Equipment and Procedure Bunsen Ice Calorimeter Computer Programs Shomate Method Treatment of Data Near the Melting Point Solution Calorimeter Heat of Solution Calculations III. RESULTS AND DISCUSSION 41 Lead Fluoride Variation in Heat Contents of the Nichrome V Capsules Lead Chloride Lead Bromide Lead Iodide Summary BIBLIOGRAPHY ...... 98 in LIST OF TABLES Table Pa8e I. High-Temperature Enthalpy Data for Cubic PbE2 Encapsulated in Gold 42 II. High-Temperature Enthalpy Data for Cubic PbF2 Encapsulated in Molybdenum 48 III. -

A Simple Method to Estimate Entropy and Free Energy of Atmospheric Gases from Their Action
Article A Simple Method to Estimate Entropy and Free Energy of Atmospheric Gases from Their Action Ivan Kennedy 1,2,*, Harold Geering 2, Michael Rose 3 and Angus Crossan 2 1 Sydney Institute of Agriculture, University of Sydney, NSW 2006, Australia 2 QuickTest Technologies, PO Box 6285 North Ryde, NSW 2113, Australia; [email protected] (H.G.); [email protected] (A.C.) 3 NSW Department of Primary Industries, Wollongbar NSW 2447, Australia; [email protected] * Correspondence: [email protected]; Tel.: + 61-4-0794-9622 Received: 23 March 2019; Accepted: 26 April 2019; Published: 1 May 2019 Abstract: A convenient practical model for accurately estimating the total entropy (ΣSi) of atmospheric gases based on physical action is proposed. This realistic approach is fully consistent with statistical mechanics, but reinterprets its partition functions as measures of translational, rotational, and vibrational action or quantum states, to estimate the entropy. With all kinds of molecular action expressed as logarithmic functions, the total heat required for warming a chemical system from 0 K (ΣSiT) to a given temperature and pressure can be computed, yielding results identical with published experimental third law values of entropy. All thermodynamic properties of gases including entropy, enthalpy, Gibbs energy, and Helmholtz energy are directly estimated using simple algorithms based on simple molecular and physical properties, without resource to tables of standard values; both free energies are measures of quantum field states and of minimal statistical degeneracy, decreasing with temperature and declining density. We propose that this more realistic approach has heuristic value for thermodynamic computation of atmospheric profiles, based on steady state heat flows equilibrating with gravity. -

THERMODYNAMIC EVALUATION of the Cu-Ti SYSTEM in VIEW of SOLID STATE AMORPHIZATION REACTIONS L
THERMODYNAMIC EVALUATION OF THE Cu-Ti SYSTEM IN VIEW OF SOLID STATE AMORPHIZATION REACTIONS L. Battezzati, M. Baricco, G. Riontino, I. Soletta To cite this version: L. Battezzati, M. Baricco, G. Riontino, I. Soletta. THERMODYNAMIC EVALUATION OF THE Cu- Ti SYSTEM IN VIEW OF SOLID STATE AMORPHIZATION REACTIONS. Journal de Physique Colloques, 1990, 51 (C4), pp.C4-79-C4-85. 10.1051/jphyscol:1990409. jpa-00230769 HAL Id: jpa-00230769 https://hal.archives-ouvertes.fr/jpa-00230769 Submitted on 1 Jan 1990 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. ~OLLOQUEDE PHYSIQUE Colloque C4, suppl6ment au 11'14, Tome 51, 15 juillet 1990 THERMODYNAMIC EVALUATION OF THE Cu-Ti SYSTEM IN VIEW OF SOLID STATE AMORPHIZATION REACTIONS L. BATTEZZATI* , M. BARICCO*~ *""" , G. RIONTINO" *"* and I. SOLETTA** * '~ipartimento di Chimica Inorganics, Chimica Fisica e Chimica dei Materiali, Universitd di Torino, Italy ""~stitutoElettrotecnico Nazionale Galileo Perraris, Torino, Italy *.* Dipartimento di Chimica, Universitd di Sassari, Italy *et* INPM, Unita' di Ricerca di Torino, Italy Resumk -La description thermodynamique du sist6me Cu-Ti est reconsideree, car les calculs he la littgrature, bien que donnant une bonne representation du diagramme de phase, ne prevoient pas la possibilit6 de l'amorphisation. -

Chapter 11 Liquids, Solids, and Phase Changes
Lecture Presentation Chapter 11 Liquids, Solids, and Phase Changes 11.1, 11.2, 11.3, 11.4, 11.15, 11.17, 11.26, 11.30, 11.32, 11.40, 11.42, 11.60, 11.82, 11.116 John E. McMurry Robert C. Fay Properties of Liquids Viscosity: The measure of a liquid’s resistance to flow (higher intermolecular force, higher viscosity) Surface Tension: The resistance of a liquid to spread out and increase its surface area (forms beads) (higher intermolecular force, higher surface tension) Properties of Liquids low intermolecular force – low viscosity, low surface tension high intermolecular force – high viscosity, high surface tension HW 11.1 Properties of Liquids high intermolecular force – high viscosity, high surface tension For the following molecules which has the higher intermolecular force, viscosity and surface tension ? (LD structure, VSEPRT, dipole of molecule) (if the molecules are in the liquid state) a. CHCl3 vs CH4 b. H2O vs H2 (changed from handout) c. N Cl3 vs N H Cl2 Phase Changes between Solids, Liquids, and Gases Phase Change (State Change): A change in the physical state but not the chemical identity of a substance Fusion (melting): solid to liquid Freezing: liquid to solid Vaporization: liquid to gas Condensation: gas to liquid Sublimation: solid to gas Deposition: gas to solid Phase Changes between Solids, Liquids, and Gases Enthalpy – add heat to system Entropy – add randomness to system Phase Changes between Solids, Liquids, and Gases Heat (Enthalpy) of Fusion (DHfusion ): The amount of energy required to overcome enough intermolecular forces to convert a solid to a liquid Heat (Enthalpy) of Vaporization (DHvap): The amount of energy required to overcome enough intermolecular forces to convert a liquid to a gas HW 11.2: Phase Changes between Solids, Liquids, & Gases DHfusion solid to a liquid DHvap liquid to a gas At phase change (melting, boiling, etc) : DG = DH – TDS & D G = zero (bc 2 phases in equilibrium) DH = TDS o a. -

Proximity to a Critical Point Driven by Electronic Entropy in Uru2si2
www.nature.com/npjquantmats ARTICLE OPEN Proximity to a critical point driven by electronic entropy in URu2Si2 ✉ Neil Harrison 1 , Satya K. Kushwaha1,2, Mun K. Chan 1 and Marcelo Jaime 1 The strongly correlated actinide metal URu2Si2 exhibits a mean field-like second order phase transition at To ≈ 17 K, yet lacks definitive signatures of a broken symmetry. Meanwhile, various experiments have also shown the electronic energy gap to closely resemble that resulting from hybridization between conduction electron and 5f-electron states. We argue here, using thermodynamic measurements, that the above seemingly incompatible observations can be jointly understood by way of proximity to an entropy-driven critical point, in which the latent heat of a valence-type electronic instability is quenched by thermal excitations across a gap, driving the transition second order. Salient features of such a transition include a robust gap spanning highly degenerate features in the electronic density of states, that is weakly (if at all) suppressed by temperature on approaching To, and an elliptical phase boundary in magnetic field and temperature that is Pauli paramagnetically limited at its critical magnetic field. npj Quantum Materials (2021) 6:24 ; https://doi.org/10.1038/s41535-021-00317-6 1234567890():,; INTRODUCTION no absolute requirement for the magnitude of a hybridization gap URu2Si2 remains of immense interest owing to the possibility to vanish at a phase transition, it need not be thermally of it exhibiting a form of order distinct from that observed -

Efficient Approach to Compute Melting Properties Fully from Ab Initio With
PHYSICAL REVIEW B 96, 224202 (2017) Efficient approach to compute melting properties fully from ab initio with application to Cu Li-Fang Zhu,* Blazej Grabowski, and Jörg Neugebauer Max-Planck-Institut für Eisenforschung GmbH, D-40237 Düsseldorf, Germany (Received 3 August 2017; revised manuscript received 16 October 2017; published 13 December 2017) Applying thermodynamic integration within an ab initio-based free-energy approach is a state-of-the-art method to calculate melting points of materials. However, the high computational cost and the reliance on a good reference system for calculating the liquid free energy have so far hindered a general application. To overcome these challenges, we propose the two-optimized references thermodynamic integration using Langevin dynamics (TOR-TILD) method in this work by extending the two-stage upsampled thermodynamic integration using Langevin dynamics (TU-TILD) method, which has been originally developed to obtain anharmonic free energies of solids, to the calculation of liquid free energies. The core idea of TOR-TILD is to fit two empirical potentials to the energies from density functional theory based molecular dynamics runs for the solid and the liquid phase and to use these potentials as reference systems for thermodynamic integration. Because the empirical potentials closely reproduce the ab initio system in the relevant part of the phase space the convergence of the thermodynamic integration is very rapid. Therefore, the proposed approach improves significantly the computational efficiency while preserving the required accuracy. As a test case, we apply TOR-TILD to fcc Cu computing not only the melting point but various other melting properties, such as the entropy and enthalpy of fusion and the volume change upon melting. -

Electronic Entropy Contribution to the Metal Insulator Transition in VO$ 2
Electronic entropy contribution to the metal insulator transition in VO2 The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Paras, J. and A. Allanore. "Electronic entropy contribution to the metal insulator transition in VO₂" Physical Review B 102, 16 (October 2020): 165138. © 2020 American Physical Society As Published http://dx.doi.org/10.1103/physrevb.102.165138 Publisher American Physical Society (APS) Version Final published version Citable link https://hdl.handle.net/1721.1/131098 Terms of Use Article is made available in accordance with the publisher's policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use. PHYSICAL REVIEW B 102, 165138 (2020) Electronic entropy contribution to the metal insulator transition in VO2 J. Paras and A. Allanore Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA (Received 11 June 2020; revised 31 August 2020; accepted 1 September 2020; published 21 October 2020) VO2 experiences a metal-insulator transition at 340 K. Discontinuities in electronic transport properties, such as the Seebeck coefficient and the electronic conductivity, suggest that there is a significant change in the electronic structure upon metallization. However, the thermodynamic nature of this transformation remains difficult to describe using conventional computational and experimental methods. This has led to disagreement over the relative importance of the change in electronic entropy with respect to the overall transition entropy. A method is presented that links measurable electronic transport properties to the change in electronic state entropy of conduction electrons. The change in electronic entropy is calculated to be 9.2 ± 0.7J/mol K which accounts for 62%–67% of the total transition entropy. -

Cryometric Determination of the Enthalpy of Fusion of Sodium Cryolite
Cryometric determination of the enthalpy of fusion of sodium cryolite M. MALINOVSKÝ Department of Chemical Technology of Inorganic Substances, Slovak Technical University, CS-812 37Bratislava Received 30 July 1982 Accepted for publication 22 August 1983 Using the method of classical thermal analysis a part of the liquidus of sodium cryolite in the system Na3AlF6—Na3FS04 was measured in the compo sition range 0—5 mole % Na3FS04. On the basis of these results the molar enthalpy of fusion of cryolite was calculated. It was found that AHf(Na3AlFft) = = 115.4 kJ mol"1; error in this result was estimated to be ±4.9 %. Методом классического термического анализа измерена часть ликви дуса натриевого криолита в системе Na3AlF6—Na3FS04 в интервале кон центраций 0—5 мол. % Na3FS04. На основании этих результатов рассчи тана мольная энтальпия плавления криолита. Найдено, что 1 AH,(Na3AlF6) = 115,4 кДж моль" ; точность этого результата ±4,9 %. Literature survey and theoretical Knowledge of the enthalpy of fusion of sodium hexafluoroaluminate Na3AlF6 (cryolite) is important both from theoretical and practical points of view. For example, it is needed at calculation of the thermal and energy balance and/or the thermal inertia of aluminium cells (the electrolyte contains about 90 mass % of Na3AlF6). The most precise values of the molar enthalpy of fusion AHf(i) of chemical substances can be generally obtained from calorimetric measurements. However, when we deal with substances having higher melting temperature and which are also unstable and very corrosive the calorimetric determination of the quantity AHf(i) can be less reliable. In the case of cryolite Roth and Bertram [1] found from the calorimetric 1 1 measurements ÄH,(Na3 A1F6) = 16.64 kcal mol" = 69.62 kJ mol" . -

Linear Dielectric Thermodynamics
Linear Dielectric Thermodynamics: A New Universal Law for Optical, Dielectric Constants by S. J. Burns Materials Science Program Department of Mechanical Engineering University of Rochester Rochester, NY 14627 USA April 17, 2020 Abstract Linear dielectric thermodynamics are formally developed to explore the isothermal and adiabatic temperature - pressure dependence of dielectric constants. The refractive index of optical materials is widely measured in the literature: it is both temperature and pressure dependent. The argument to establish the dielectric constant’s isentropic temperature dependence is a thermodynamic one and is thus applicable to all physical models that describe electron clouds and electronic resonances within materials. The isentropic slope of the displacement field versus the electric field at all temperatures is described by an adiabatic dielectric constant in an energy- per-unit mass system. This slope is shown through the electronic part of the entropy to be unstable at high temperatures due to the change in the curvature of the temperature dependence of the dielectric constant. The electronic entropy contribution for optical, thermo-electro materials has negative heat capacities which are unacceptable. The dielectric constant’s temperature and pressure dependence is predicted to be only dependent on the specific volume so isentropes are always positive. A new universal form for the dielectric constant follows from this hypothesis: the dielectric constant is proportional to the square root of the specific volume.