Msan(2020)32
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent Office
3,092,548 United States Patent Office Patented June 4, 1963 2 are the various side effects that occur in an appreciable 3,092,548 number of patients, the foremost of which are a blurring METHOD OF TREATING PEPTICULCERS WITH PANTOTHENECACD of vision, drowsiness and a general dry condition mani Albert G. Worton, Columbus, Ohio, assignor to The fested by a retarded salivation, reduction of perspiration Warren-Teed Products Company, Columbus, Ohio, a 5 and diminished urinary output. Other side effects which corporation of Ohio occur in some cases are glaucoma, stimulation of the cen No Drawing. Filed Oct. 27, 1960, Ser. No. 65,256 trol nervous system and in severe cases cardiac and respi 5 Claims. (Ci. 67-55) ratory collapse. These side effects increase to Some de gree with the increase in dosage. Despite the side effects This invention relates to preparation adapted for use O these compounds are fairly selective and highly effective in treating disorders of the gastro-intestinal tract, more in decreasing the volume and acidity of gastric secretion particularly in treating peptic ulcer, both of the duodenal and in reducing gastrointestinal motility. and gastric type, for hyperacidity, hypertropic gastritis, Understandably, the main problem in the past has been splenic flexure syndrome, biliary dyskinesia (postchole to provide a dosage of anticholinergic drug which will cystectomy syndrome) and hiatal hernia or other condi achieve the most beneficial results possible and yet mini tions where anticholinergic effect, either spasmolytic or mize the undesired side effects. One of the objects of antisecretory is indicated or where antiuicerogenic effect this invention is to provide novel compositions containing is indicated. -

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Norfolk & Waveney Prescriber
The Norfolk & Waveney Prescriber Issue 119 April 2017 For Primary and Secondary Care Health Professionals in Norfolk & Waveney In this issue: Prescribing Safety News: MHRA Drug Safety Updates 2 TAG and Commissioning News 3-4 Drugs – discontinued, shortages and prices 5-6 Drugs – supply issues 7-8 Guidance and Policy News: - National: “NICE Bites” & NICE guidance 9-10 Guidance and Policy News: - Local: Key Message Bulletins 11-12 Cost-effective Prescribing Tips: 13 Ophthalmic Special Order products Atorvastatin Hot Topic: Multimorbidity and Polypharmacy 14 Prescribing Safety: Medicines and Serotonin Syndrome 15-16 Prescribing Advice: Metformin, Finasteride, and Inhalers 17-18 New Medicines & Indications News 19 Feedback and comments on this edition to [email protected] / 01603 257035 Prescribing & Medicines Management Team – 1 NEL CSU Anglia Prescribing Safety News Main points from MHRA Drug Safety Updates February - March 2017 Articles relevant to Primary Care and Secondary Care: SGLT2 inhibitors: updated advice on increased risk of lower-limb amputation (mainly toes) Canagliflozin may increase the risk of lower-limb amputation (mainly toes) in patients with type 2 diabetes. Evidence does not show an increased risk for dapagliflozin and empagliflozin, but the risk may be a class effect. Preventive foot care is important for all patients with diabetes. Advice for healthcare professionals: o carefully monitor patients receiving canagliflozin who have risk factors for amputation, such as poor control of diabetes and problems with -

Muscarinic Acetylcholine Receptor
mAChR Muscarinic acetylcholine receptor mAChRs (muscarinic acetylcholine receptors) are acetylcholine receptors that form G protein-receptor complexes in the cell membranes of certainneurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibersin the parasympathetic nervous system. mAChRs are named as such because they are more sensitive to muscarine than to nicotine. Their counterparts are nicotinic acetylcholine receptors (nAChRs), receptor ion channels that are also important in the autonomic nervous system. Many drugs and other substances (for example pilocarpineand scopolamine) manipulate these two distinct receptors by acting as selective agonists or antagonists. Acetylcholine (ACh) is a neurotransmitter found extensively in the brain and the autonomic ganglia. www.MedChemExpress.com 1 mAChR Inhibitors & Modulators (+)-Cevimeline hydrochloride hemihydrate (-)-Cevimeline hydrochloride hemihydrate Cat. No.: HY-76772A Cat. No.: HY-76772B Bioactivity: Cevimeline hydrochloride hemihydrate, a novel muscarinic Bioactivity: Cevimeline hydrochloride hemihydrate, a novel muscarinic receptor agonist, is a candidate therapeutic drug for receptor agonist, is a candidate therapeutic drug for xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR The general pharmacol. properties of this drug on the The general pharmacol. properties of this drug on the gastrointestinal, urinary, and reproductive systems and other… gastrointestinal, urinary, and reproductive systems and other… Purity: >98% Purity: >98% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 10mM x 1mL in DMSO, Size: 10mM x 1mL in DMSO, 1 mg, 5 mg 1 mg, 5 mg AC260584 Aclidinium Bromide Cat. No.: HY-100336 (LAS 34273; LAS-W 330) Cat. -

Reference List of Drugs with Potential Anticholinergic Effects 1, 2, 3, 4, 5
ANTICHOLINERGICS: Reference List of Drugs with Potential Anticholinergic Effects 1, 2, 3, 4, 5 J Bareham BSP © www.RxFiles.ca Aug 2021 WHENEVER POSSIBLE, AVOID DRUGS WITH MODERATE TO HIGH ANTICHOLINERGIC ACTIVITY IN OLDER ADULTS (>65 YEARS OF AGE) Low Anticholinergic Activity; Moderate/High Anticholinergic Activity -B in combo Beers Antibiotics Antiparkinsonian Cardiovascular Agents Immunosuppressants ampicillin *ALL AVAILABLE AS amantadine SYMMETREL atenolol TENORMIN azaTHIOprine IMURAN cefOXitin GENERIC benztropine mesylate COGENTIN captopril CAPOTEN cyclosporine NEORAL clindamycin bromocriptine PARLODEL chlorthalidone GENERIC ONLY hydrocortisone CORTEF gentamicin (Oint & Sol’n NIHB covered) carbidopa/levodopa SINEMET digoxin LANOXIN, TOLOXIN methylprednisolone MEDROL piperacillin entacapone COMTAN dilTIAZem CARDIZEM, TIAZAC prednisone WINPRED dipyridamole PERSANTINE, ethopropazine PARSITAN vancomycin phenelzine NARDIL AGGRENOX disopyramide RYTHMODAN Muscle Relaxants pramipexole MIRAPEX Antidepressants baclofen LIORESAL ( on intrathecal only) procyclidine KEMADRIN furosemide LASIX amitriptyline ELAVIL cyclobenzaprine FLEXERIL selegiline ELDEPRYL hydrALAZINE APRESOLINE clomiPRAMINE ANAFRANIL isosorbide ISORDIL methocarbamol ROBAXIN OTC trihexyphenidyl ARTANE desipramine NORPRAMIN metoprolol LOPRESOR orphenadrine NORFLEX OTC doxepin >6mg SINEQUAN Antipsychotics NIFEdipine ADALAT tiZANidine ZANAFLEX A imipramine TOFRANIL quiNIDine GENERIC ONLY C ARIPiprazole ABILIFY & MAINTENA -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

Effect of Diazepam and Hyoscine Butylbromide on Response to Secretin and Cholecystokinin-Pancreozymin in Man
Gut: first published as 10.1136/gut.17.5.351 on 1 May 1976. Downloaded from Gut, 1976, 17, 351-353 Effect of diazepam and hyoscine butylbromide on response to secretin and cholecystokinin-pancreozymin in man J. H. B. SAUNDERS, GUYA MASOERO, AND K. G. WORMSLEY' From the Department of Therapeutics, University of Dundee, Dundee SUMMARY Ten subjects received secretin and cholecystokinin or, in duplicate tests, the two hor- mones together with either diazepam or diazepam plus hyoscine butylbromide in order to determine whether these drugs, which are often used during retrograde endoscopic cannulation ofthe pancreatic duct, affect pancreatic and biliary secretion in response to the hormones. Diazepam with hyoscine butylbromide reduced the secretion of trypsin into the duodenum and delayed the appearance of both trypsin and bilirubin in duodenal aspirate. These effects must be taken into account when interpreting pancreatic and biliary responses measured during direct cannulation of the pancreatic duct. Endoscopic retrograde cannulation of the pancreatic vents in the antrum. Secretin (1 clinical unit/kg-h) duct has recently been used to measure pancreatic and cholecystokinin-pancreozymin (1 Ivy unit/ function by collecting the pancreatic juice directly kg-h) were administered for 45 minutes by continuous from the pancreatic duct during stimulation with intravenous infusion in 0 15 mol/1 sodium chloride. hormones et The exogenous (Cotton al., 1974; Gregg hormones had been purchased from the GIH http://gut.bmj.com/ et al., 1975; Robberecht et -

PROPANTHELINE BROMIDE Tablets USP Rx Only DESCRIPTION
PROPANTHELINE BROMIDE Tablets USP PRODUCT OVERVIEW: Propantheline Bromide TABLET, FILM COATED Rx only DESCRIPTION Each tablet for oral administration contains: Propantheline bromide.................................................. 15 mg Propantheline bromide, a synthetic quaternary ammonium compound, occurs as white or nearly white crystals. It is odorless and has a bitter taste, and is very soluble in water and chloroform; practically insoluble in ether, acetone and ethyl acetate. It is designated chemically as (2- Hydroxyethyl) diisopropylmethyl-ammonium bromide xanthene-9-carboxylate. The structural formula is: Inactive Ingredients The tablets contain hydroxypropyl methylcellulose, lactose, magnesium stearate, starch, sterotex, and other ingredients. CLINICAL PHARMACOLOGY Propantheline bromide inhibits gastrointestinal motility and diminishes gastric acid secretion. The drug also inhibits the action of acetylcholine at the postganglionic nerve endings of the parasympathetic nervous system. Propantheline bromide is extensively metabolized in man primarily by hydrolysis to the inactive materials xanthene-9-carboxylic acid and (2-hydroxyethyl) diisopropylmethylammonium bromide. In a bioavailability study, peak plasma concentrations of propantheline were achieved in about one hour, following a single oral dose. The plasma elimination half-life of propantheline is about 1.6 hours. Approximately 70% of the dose is excreted in the urine, mostly as metabolites. The urinary excretion of propantheline is about 3% after oral tablet administration. INDICATIONS AND USAGE Propantheline bromide is effective as adjunctive therapy in the treatment of peptic ulcer. CONTRAINDICATIONS Propantheline is contraindicated in patients with: 1. Glaucoma, since mydriasis is to be avoided. 2. Obstructive disease of the gastrointestinal tract (pyloroduodenal stenosis, achalasia, paralytic ileus, etc.). 3. Obstructive uropathy (e.g., bladder-neck obstruction due to prostatic hypertrophy). -
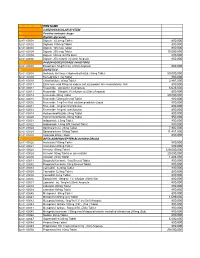
National Code Item Name 1
NATIONAL CODE ITEM NAME 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1AA Digtalis glycoside 02-01-00001 Digoxin 62.5mcg Tablet 800,000 02-01-00002 Digitoxin 100mcg Tablet 800,000 02-01-00003 Digoxin 125 mcg Tablet 800,000 02-01-00004 Digoxin 250 mcg Tablet 15,000,000 02-01-00005 Digoxin 50mcg /ml PG Elixir 800,000 02-01-00006 Digoxin 250 mcg/ml inj (2ml) Ampoule 800,000 1AB PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone 5mg/1ml inj (20ml) Ampoule 800,000 1B DIURETICS 02-01-00008 Amiloride Hcl 5mg + Hydrochlorthiazide 50mg Tablet 50,000,000 02-01-00009 Bumetanide 1 mg Tablet 800,000 02-01-00010 Chlorthalidone 50mg Tablet 2,867,000 02-01-00011 Ethacrynic acid 50mg as sodium salt inj (powder for reconstitution) Vial 800,000 02-01-00012 Frusemide 20mg/2ml inj Ampoule 6,625,000 02-01-00013 Frusemide 10mg/ml,I.V.infusion inj (25ml) Ampoule 800,000 02-01-00014 Frusemide 40mg Tablet 20,000,000 02-01-00015 Frusemide 500mg Scored Tablet 800,000 02-01-00016 Frusemide 1mg/1ml Oral solution peadiatric Liquid 800,000 02-01-00017 Frusemide 4mg/ml Oral Solution 800,000 02-01-00018 Frusemide 8mg/ml oral Solution 800,000 02-01-00019 Hydrochlorothiazide 25mg Tablet 800,000 02-01-00020 Hydrochlorothiazide 50mg Tablet 950,000 02-01-00021 Indapamide 2.5mg Tablet 800,000 02-01-00022 Indapamide 1.5mg S/R Coated Tablet 800,000 02-01-00023 Spironolactone 25mg Tablet 7,902,000 02-01-00024 Spironolactone 100mg Tablet 11,451,000 02-01-00025 Xipamide 20mg Tablet 800,000 1C BETA-ADRENOCEPTER BLOCKING DRUGS 02-01-00026 Acebutolol 100mg Tablet 800,000 02-01-00027 Acebutolol 200mg Tablet 800,000 02-01-00028 Atenolol 100mg Tablet 120,000,000 02-01-00029 Atenolol 50mg Tablet or (scored tab) 20,000,000 02-01-00030 Atenolol 25mg Tablet 1,483,000 02-01-00031 Bisoprolol fumarate 5mg Scored Tablet 800,000 02-01-00032 Bisoprolol fumarate 10mg Scored Tablet 800,000 02-01-00033 Carvedilol 6.25mg Tablet 800,000 02-01-00034 Carvedilol 12.5mg Tablet 800,000 02-01-00035 Carvedilol 25mg Tablet 800,000 02-01-00036 Esmolol Hcl 10mg/ml I.V. -

1: Gastro-Intestinal System
1 1: GASTRO-INTESTINAL SYSTEM Antacids .......................................................... 1 Stimulant laxatives ...................................46 Compound alginate products .................. 3 Docuate sodium .......................................49 Simeticone ................................................... 4 Lactulose ....................................................50 Antimuscarinics .......................................... 5 Macrogols (polyethylene glycols) ..........51 Glycopyrronium .......................................13 Magnesium salts ........................................53 Hyoscine butylbromide ...........................16 Rectal products for constipation ..........55 Hyoscine hydrobromide .........................19 Products for haemorrhoids .................56 Propantheline ............................................21 Pancreatin ...................................................58 Orphenadrine ...........................................23 Prokinetics ..................................................24 Quick Clinical Guides: H2-receptor antagonists .......................27 Death rattle (noisy rattling breathing) 12 Proton pump inhibitors ........................30 Opioid-induced constipation .................42 Loperamide ................................................35 Bowel management in paraplegia Laxatives ......................................................38 and tetraplegia .....................................44 Ispaghula (Psyllium husk) ........................45 ANTACIDS Indications: -

(12) Patent Application Publication (10) Pub. No.: US 2006/0024365A1 Vaya Et Al
US 2006.0024.365A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0024365A1 Vaya et al. (43) Pub. Date: Feb. 2, 2006 (54) NOVEL DOSAGE FORM (30) Foreign Application Priority Data (76) Inventors: Navin Vaya, Gujarat (IN); Rajesh Aug. 5, 2002 (IN)................................. 699/MUM/2002 Singh Karan, Gujarat (IN); Sunil Aug. 5, 2002 (IN). ... 697/MUM/2002 Sadanand, Gujarat (IN); Vinod Kumar Jan. 22, 2003 (IN)................................... 80/MUM/2003 Gupta, Gujarat (IN) Jan. 22, 2003 (IN)................................... 82/MUM/2003 Correspondence Address: Publication Classification HEDMAN & COSTIGAN P.C. (51) Int. Cl. 1185 AVENUE OF THE AMERICAS A6IK 9/22 (2006.01) NEW YORK, NY 10036 (US) (52) U.S. Cl. .............................................................. 424/468 (22) Filed: May 19, 2005 A dosage form comprising of a high dose, high Solubility active ingredient as modified release and a low dose active ingredient as immediate release where the weight ratio of Related U.S. Application Data immediate release active ingredient and modified release active ingredient is from 1:10 to 1:15000 and the weight of (63) Continuation-in-part of application No. 10/630,446, modified release active ingredient per unit is from 500 mg to filed on Jul. 29, 2003. 1500 mg, a process for preparing the dosage form. Patent Application Publication Feb. 2, 2006 Sheet 1 of 10 US 2006/0024.365A1 FIGURE 1 FIGURE 2 FIGURE 3 Patent Application Publication Feb. 2, 2006 Sheet 2 of 10 US 2006/0024.365A1 FIGURE 4 (a) 7 FIGURE 4 (b) Patent Application Publication Feb. 2, 2006 Sheet 3 of 10 US 2006/0024.365 A1 FIGURE 5 100 ov -- 60 40 20 C 2 4.