New Zealand Data Sheet 1 Product Name 2 Qualitative
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

United States Patent Office
3,092,548 United States Patent Office Patented June 4, 1963 2 are the various side effects that occur in an appreciable 3,092,548 number of patients, the foremost of which are a blurring METHOD OF TREATING PEPTICULCERS WITH PANTOTHENECACD of vision, drowsiness and a general dry condition mani Albert G. Worton, Columbus, Ohio, assignor to The fested by a retarded salivation, reduction of perspiration Warren-Teed Products Company, Columbus, Ohio, a 5 and diminished urinary output. Other side effects which corporation of Ohio occur in some cases are glaucoma, stimulation of the cen No Drawing. Filed Oct. 27, 1960, Ser. No. 65,256 trol nervous system and in severe cases cardiac and respi 5 Claims. (Ci. 67-55) ratory collapse. These side effects increase to Some de gree with the increase in dosage. Despite the side effects This invention relates to preparation adapted for use O these compounds are fairly selective and highly effective in treating disorders of the gastro-intestinal tract, more in decreasing the volume and acidity of gastric secretion particularly in treating peptic ulcer, both of the duodenal and in reducing gastrointestinal motility. and gastric type, for hyperacidity, hypertropic gastritis, Understandably, the main problem in the past has been splenic flexure syndrome, biliary dyskinesia (postchole to provide a dosage of anticholinergic drug which will cystectomy syndrome) and hiatal hernia or other condi achieve the most beneficial results possible and yet mini tions where anticholinergic effect, either spasmolytic or mize the undesired side effects. One of the objects of antisecretory is indicated or where antiuicerogenic effect this invention is to provide novel compositions containing is indicated. -

Muscarinic Acetylcholine Receptor
mAChR Muscarinic acetylcholine receptor mAChRs (muscarinic acetylcholine receptors) are acetylcholine receptors that form G protein-receptor complexes in the cell membranes of certainneurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibersin the parasympathetic nervous system. mAChRs are named as such because they are more sensitive to muscarine than to nicotine. Their counterparts are nicotinic acetylcholine receptors (nAChRs), receptor ion channels that are also important in the autonomic nervous system. Many drugs and other substances (for example pilocarpineand scopolamine) manipulate these two distinct receptors by acting as selective agonists or antagonists. Acetylcholine (ACh) is a neurotransmitter found extensively in the brain and the autonomic ganglia. www.MedChemExpress.com 1 mAChR Inhibitors & Modulators (+)-Cevimeline hydrochloride hemihydrate (-)-Cevimeline hydrochloride hemihydrate Cat. No.: HY-76772A Cat. No.: HY-76772B Bioactivity: Cevimeline hydrochloride hemihydrate, a novel muscarinic Bioactivity: Cevimeline hydrochloride hemihydrate, a novel muscarinic receptor agonist, is a candidate therapeutic drug for receptor agonist, is a candidate therapeutic drug for xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR xerostomia in Sjogren's syndrome. IC50 value: Target: mAChR The general pharmacol. properties of this drug on the The general pharmacol. properties of this drug on the gastrointestinal, urinary, and reproductive systems and other… gastrointestinal, urinary, and reproductive systems and other… Purity: >98% Purity: >98% Clinical Data: No Development Reported Clinical Data: No Development Reported Size: 10mM x 1mL in DMSO, Size: 10mM x 1mL in DMSO, 1 mg, 5 mg 1 mg, 5 mg AC260584 Aclidinium Bromide Cat. No.: HY-100336 (LAS 34273; LAS-W 330) Cat. -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -

Effect of Diazepam and Hyoscine Butylbromide on Response to Secretin and Cholecystokinin-Pancreozymin in Man
Gut: first published as 10.1136/gut.17.5.351 on 1 May 1976. Downloaded from Gut, 1976, 17, 351-353 Effect of diazepam and hyoscine butylbromide on response to secretin and cholecystokinin-pancreozymin in man J. H. B. SAUNDERS, GUYA MASOERO, AND K. G. WORMSLEY' From the Department of Therapeutics, University of Dundee, Dundee SUMMARY Ten subjects received secretin and cholecystokinin or, in duplicate tests, the two hor- mones together with either diazepam or diazepam plus hyoscine butylbromide in order to determine whether these drugs, which are often used during retrograde endoscopic cannulation ofthe pancreatic duct, affect pancreatic and biliary secretion in response to the hormones. Diazepam with hyoscine butylbromide reduced the secretion of trypsin into the duodenum and delayed the appearance of both trypsin and bilirubin in duodenal aspirate. These effects must be taken into account when interpreting pancreatic and biliary responses measured during direct cannulation of the pancreatic duct. Endoscopic retrograde cannulation of the pancreatic vents in the antrum. Secretin (1 clinical unit/kg-h) duct has recently been used to measure pancreatic and cholecystokinin-pancreozymin (1 Ivy unit/ function by collecting the pancreatic juice directly kg-h) were administered for 45 minutes by continuous from the pancreatic duct during stimulation with intravenous infusion in 0 15 mol/1 sodium chloride. hormones et The exogenous (Cotton al., 1974; Gregg hormones had been purchased from the GIH http://gut.bmj.com/ et al., 1975; Robberecht et -

PROPANTHELINE BROMIDE Tablets USP Rx Only DESCRIPTION
PROPANTHELINE BROMIDE Tablets USP PRODUCT OVERVIEW: Propantheline Bromide TABLET, FILM COATED Rx only DESCRIPTION Each tablet for oral administration contains: Propantheline bromide.................................................. 15 mg Propantheline bromide, a synthetic quaternary ammonium compound, occurs as white or nearly white crystals. It is odorless and has a bitter taste, and is very soluble in water and chloroform; practically insoluble in ether, acetone and ethyl acetate. It is designated chemically as (2- Hydroxyethyl) diisopropylmethyl-ammonium bromide xanthene-9-carboxylate. The structural formula is: Inactive Ingredients The tablets contain hydroxypropyl methylcellulose, lactose, magnesium stearate, starch, sterotex, and other ingredients. CLINICAL PHARMACOLOGY Propantheline bromide inhibits gastrointestinal motility and diminishes gastric acid secretion. The drug also inhibits the action of acetylcholine at the postganglionic nerve endings of the parasympathetic nervous system. Propantheline bromide is extensively metabolized in man primarily by hydrolysis to the inactive materials xanthene-9-carboxylic acid and (2-hydroxyethyl) diisopropylmethylammonium bromide. In a bioavailability study, peak plasma concentrations of propantheline were achieved in about one hour, following a single oral dose. The plasma elimination half-life of propantheline is about 1.6 hours. Approximately 70% of the dose is excreted in the urine, mostly as metabolites. The urinary excretion of propantheline is about 3% after oral tablet administration. INDICATIONS AND USAGE Propantheline bromide is effective as adjunctive therapy in the treatment of peptic ulcer. CONTRAINDICATIONS Propantheline is contraindicated in patients with: 1. Glaucoma, since mydriasis is to be avoided. 2. Obstructive disease of the gastrointestinal tract (pyloroduodenal stenosis, achalasia, paralytic ileus, etc.). 3. Obstructive uropathy (e.g., bladder-neck obstruction due to prostatic hypertrophy). -
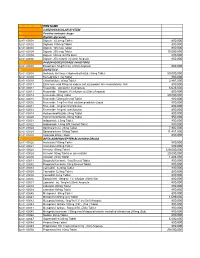
National Code Item Name 1
NATIONAL CODE ITEM NAME 1 CARDIOVASCULAR SYSTEM 1A Positive inotropic drugs 1AA Digtalis glycoside 02-01-00001 Digoxin 62.5mcg Tablet 800,000 02-01-00002 Digitoxin 100mcg Tablet 800,000 02-01-00003 Digoxin 125 mcg Tablet 800,000 02-01-00004 Digoxin 250 mcg Tablet 15,000,000 02-01-00005 Digoxin 50mcg /ml PG Elixir 800,000 02-01-00006 Digoxin 250 mcg/ml inj (2ml) Ampoule 800,000 1AB PHOSPHODIESTERASE INHIBITORS 02-01-00007 Enoximone 5mg/1ml inj (20ml) Ampoule 800,000 1B DIURETICS 02-01-00008 Amiloride Hcl 5mg + Hydrochlorthiazide 50mg Tablet 50,000,000 02-01-00009 Bumetanide 1 mg Tablet 800,000 02-01-00010 Chlorthalidone 50mg Tablet 2,867,000 02-01-00011 Ethacrynic acid 50mg as sodium salt inj (powder for reconstitution) Vial 800,000 02-01-00012 Frusemide 20mg/2ml inj Ampoule 6,625,000 02-01-00013 Frusemide 10mg/ml,I.V.infusion inj (25ml) Ampoule 800,000 02-01-00014 Frusemide 40mg Tablet 20,000,000 02-01-00015 Frusemide 500mg Scored Tablet 800,000 02-01-00016 Frusemide 1mg/1ml Oral solution peadiatric Liquid 800,000 02-01-00017 Frusemide 4mg/ml Oral Solution 800,000 02-01-00018 Frusemide 8mg/ml oral Solution 800,000 02-01-00019 Hydrochlorothiazide 25mg Tablet 800,000 02-01-00020 Hydrochlorothiazide 50mg Tablet 950,000 02-01-00021 Indapamide 2.5mg Tablet 800,000 02-01-00022 Indapamide 1.5mg S/R Coated Tablet 800,000 02-01-00023 Spironolactone 25mg Tablet 7,902,000 02-01-00024 Spironolactone 100mg Tablet 11,451,000 02-01-00025 Xipamide 20mg Tablet 800,000 1C BETA-ADRENOCEPTER BLOCKING DRUGS 02-01-00026 Acebutolol 100mg Tablet 800,000 02-01-00027 Acebutolol 200mg Tablet 800,000 02-01-00028 Atenolol 100mg Tablet 120,000,000 02-01-00029 Atenolol 50mg Tablet or (scored tab) 20,000,000 02-01-00030 Atenolol 25mg Tablet 1,483,000 02-01-00031 Bisoprolol fumarate 5mg Scored Tablet 800,000 02-01-00032 Bisoprolol fumarate 10mg Scored Tablet 800,000 02-01-00033 Carvedilol 6.25mg Tablet 800,000 02-01-00034 Carvedilol 12.5mg Tablet 800,000 02-01-00035 Carvedilol 25mg Tablet 800,000 02-01-00036 Esmolol Hcl 10mg/ml I.V. -

1: Gastro-Intestinal System
1 1: GASTRO-INTESTINAL SYSTEM Antacids .......................................................... 1 Stimulant laxatives ...................................46 Compound alginate products .................. 3 Docuate sodium .......................................49 Simeticone ................................................... 4 Lactulose ....................................................50 Antimuscarinics .......................................... 5 Macrogols (polyethylene glycols) ..........51 Glycopyrronium .......................................13 Magnesium salts ........................................53 Hyoscine butylbromide ...........................16 Rectal products for constipation ..........55 Hyoscine hydrobromide .........................19 Products for haemorrhoids .................56 Propantheline ............................................21 Pancreatin ...................................................58 Orphenadrine ...........................................23 Prokinetics ..................................................24 Quick Clinical Guides: H2-receptor antagonists .......................27 Death rattle (noisy rattling breathing) 12 Proton pump inhibitors ........................30 Opioid-induced constipation .................42 Loperamide ................................................35 Bowel management in paraplegia Laxatives ......................................................38 and tetraplegia .....................................44 Ispaghula (Psyllium husk) ........................45 ANTACIDS Indications: -

(12) Patent Application Publication (10) Pub. No.: US 2006/0024365A1 Vaya Et Al
US 2006.0024.365A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0024365A1 Vaya et al. (43) Pub. Date: Feb. 2, 2006 (54) NOVEL DOSAGE FORM (30) Foreign Application Priority Data (76) Inventors: Navin Vaya, Gujarat (IN); Rajesh Aug. 5, 2002 (IN)................................. 699/MUM/2002 Singh Karan, Gujarat (IN); Sunil Aug. 5, 2002 (IN). ... 697/MUM/2002 Sadanand, Gujarat (IN); Vinod Kumar Jan. 22, 2003 (IN)................................... 80/MUM/2003 Gupta, Gujarat (IN) Jan. 22, 2003 (IN)................................... 82/MUM/2003 Correspondence Address: Publication Classification HEDMAN & COSTIGAN P.C. (51) Int. Cl. 1185 AVENUE OF THE AMERICAS A6IK 9/22 (2006.01) NEW YORK, NY 10036 (US) (52) U.S. Cl. .............................................................. 424/468 (22) Filed: May 19, 2005 A dosage form comprising of a high dose, high Solubility active ingredient as modified release and a low dose active ingredient as immediate release where the weight ratio of Related U.S. Application Data immediate release active ingredient and modified release active ingredient is from 1:10 to 1:15000 and the weight of (63) Continuation-in-part of application No. 10/630,446, modified release active ingredient per unit is from 500 mg to filed on Jul. 29, 2003. 1500 mg, a process for preparing the dosage form. Patent Application Publication Feb. 2, 2006 Sheet 1 of 10 US 2006/0024.365A1 FIGURE 1 FIGURE 2 FIGURE 3 Patent Application Publication Feb. 2, 2006 Sheet 2 of 10 US 2006/0024.365A1 FIGURE 4 (a) 7 FIGURE 4 (b) Patent Application Publication Feb. 2, 2006 Sheet 3 of 10 US 2006/0024.365 A1 FIGURE 5 100 ov -- 60 40 20 C 2 4. -

Federal Register/Vol. 77, No. 115/Thursday, June 14, 2012
Federal Register / Vol. 77, No. 115 / Thursday, June 14, 2012 / Notices 35691 TABLE 1—LIST OF SAFETY AND EFFECTIVENESS SUMMARIES FOR APPROVED PMAS MADE AVAILABLE FROM JANUARY 1, 2012, THROUGH MARCH 31, 2012—Continued PMA No., Docket No. Applicant Trade name Approval date P060008.S046, FDA–2012–M–0210 ... Boston Scientific Corp ......................... TAXUS Liberte´ Paclitaxel-Eluting Cor- February 22, 2012. onary Stent System (Monorail and Over-The-Wire Delivery Systems). P030025.S086, FDA–2012–M–0209 ... Boston Scientific Corp ......................... TAXUS Express2 Paclitaxel-Eluting February 22, 2012. Coronary Stent System (Monorail and Over-The-Wire Delivery Sys- tems). P110023, FDA–2012–M–0221 ............ ev3, Inc ................................................ Everflex Self-Expanding Peripheral March 7, 2012. Stent System (Everflex). P070004, FDA–2012–M–0250............ Sientra, Inc.......................................... SIENTRA Silicone Gel Breast Im- March 9, 2012. plants. II. Electronic Access LOCATION: The meeting will be held at submissions. In the process of Persons with access to the Internet the FDA White Oak Campus, 10903 considering these changes, FDA has may obtain the documents at http:// New Hampshire Ave., Bldg. 31 previously made available for comment www.fda.gov/MedicalDevices/ Conference Center, Great Room 1503, versions of documents that support ProductsandMedicalProcedures/ Silver Spring, MD 20993. The following making regulatory submissions in DeviceApprovalsandClearances/ link contains public meeting attendee electronic format using the (eCTD) information as well as frequently asked PMAApprovals/default.htm and http:// specifications. These draft documents questions and answers regarding public www.fda.gov/MedicalDevices/ represented FDA’s major updates to meetings at White Oak: http:// ProductsandMedicalProcedures/ Module 1 of the eCTD based on www.fda.gov/AboutFDA/ DeviceApprovalsandClearances/ previous comments. -

Propantheline
Drug notes Propantheline James Barry1 MBBS, MRes, Foundation Doctor Sympathetic preganglionic Sympathetic postganglionic neuron (cholinergic) neuron (cholinergic) Gerry McKay1 BSc(Hons), FRCP, Consultant Physician Miles Fisher1 MD, FRCP, Consultant Physician 1Glasgow Royal Infirmary, Glasgow, UK Acetylcholine Correspondence to: Dr James Barry, Foundation Doctor, Department of Acetylcholine Diabetes, Endocrinology & Clinical Pharmacology, Propantheline Glasgow Royal Infirmary, 84 Castle Street, Glasgow G4 0SF, UK; email: [email protected] Muscarinic receptors Sweat gland Figure 1. Propantheline acts as an antagonist to acetylcholine at the muscarinic receptor within the sweat gland Introduction has a direct musculotropic effect Sweating, or diaphoresis, is an essen- causing relaxation of smooth muscle. tial mechanism of thermoregulation. Propantheline is the isopropyl ana- Aberrant diaphoresis is a recognised logue of methantheline and has up complication of diabetic autonomic to five times the antimuscarinic neuropathy which is manifest at the activity, a longer duration of action time of eating (gustatory sweating). and a smaller side effect profile. This has potentially debilitating phys- Propantheline undergoes exten- iological, social and psychological sive first pass metabolism, reducing effects and is difficult to treat. If bioavailability significantly to between avoidance of triggering foods does 10–25%. Metabolism is mainly via not improve symptoms, a trial of hydrolysis with studies demonstrating drug therapy may be indicated. peak plasma levels at 2 hours and Propantheline has been used for elimination half-life between 2–3 many years to treat gustatory sweat- hours post single-dose administration. ing, but its effectiveness compared to The pharmacological effect is seen at more novel agents and multi-modal- 1 hour and persists until 6 hours post ity therapies is uncertain.1 dose. -

Nerve Disease and Bladder Control
Nerve Disease and Bladder Control National Kidney and Urologic Diseases Information Clearinghouse For the urinary system to do its job, muscles and nerves must work together to hold Brain urine in the bladder and then release it at the right time. Nerves carry messages from NATIONAL the bladder to the brain to let it know when INSTITUTES the bladder is full. They also carry messages OF HEALTH Central nervous from the brain to the bladder, telling muscles system (brain either to tighten or release. A nerve prob and spinal cord) lem might affect your bladder control if the nerves that are supposed to carry messages Spinal cord between the brain and the bladder do not work properly. Nerve signals U.S. Department to bladder of Health and Bladder and sphincter Human Services What bladder control muscles problems does nerve damage cause? Nerves that work poorly can lead to three Urethra different kinds of bladder control problems. Overactive bladder. Damaged nerves may Sphincter muscles send signals to the bladder at the wrong time, causing its muscles to squeeze with Nerves carry signals from the brain to the bladder out warning. The symptoms of overactive and sphincter. bladder include • urinary frequency—defined as urination eight or more times a day or two or nerves to the sphincter muscles are dam more times at night aged, the muscles may become loose and allow leakage or stay tight when you are • urinary urgency—the sudden, strong trying to release urine. need to urinate immediately Urine retention. For some people, nerve • urge incontinence—leakage of urine damage means their bladder muscles do that follows a sudden, strong urge to not get the message that it is time to release urinate urine or are too weak to completely empty Poor control of sphincter muscles.