Bacterial Community, Influencing Factors, and Potential Health Effects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Characterization of the Aerobic Anoxygenic Phototrophic Bacterium Sphingomonas Sp
microorganisms Article Characterization of the Aerobic Anoxygenic Phototrophic Bacterium Sphingomonas sp. AAP5 Karel Kopejtka 1 , Yonghui Zeng 1,2, David Kaftan 1,3 , Vadim Selyanin 1, Zdenko Gardian 3,4 , Jürgen Tomasch 5,† , Ruben Sommaruga 6 and Michal Koblížek 1,* 1 Centre Algatech, Institute of Microbiology, Czech Academy of Sciences, 379 81 Tˇreboˇn,Czech Republic; [email protected] (K.K.); [email protected] (Y.Z.); [email protected] (D.K.); [email protected] (V.S.) 2 Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, 1871 Frederiksberg C, Denmark 3 Faculty of Science, University of South Bohemia, 370 05 Ceskˇ é Budˇejovice,Czech Republic; [email protected] 4 Institute of Parasitology, Biology Centre, Czech Academy of Sciences, 370 05 Ceskˇ é Budˇejovice,Czech Republic 5 Research Group Microbial Communication, Technical University of Braunschweig, 38106 Braunschweig, Germany; [email protected] 6 Laboratory of Aquatic Photobiology and Plankton Ecology, Department of Ecology, University of Innsbruck, 6020 Innsbruck, Austria; [email protected] * Correspondence: [email protected] † Present Address: Department of Molecular Bacteriology, Helmholtz-Centre for Infection Research, 38106 Braunschweig, Germany. Abstract: An aerobic, yellow-pigmented, bacteriochlorophyll a-producing strain, designated AAP5 Citation: Kopejtka, K.; Zeng, Y.; (=DSM 111157=CCUG 74776), was isolated from the alpine lake Gossenköllesee located in the Ty- Kaftan, D.; Selyanin, V.; Gardian, Z.; rolean Alps, Austria. Here, we report its description and polyphasic characterization. Phylogenetic Tomasch, J.; Sommaruga, R.; Koblížek, analysis of the 16S rRNA gene showed that strain AAP5 belongs to the bacterial genus Sphingomonas M. Characterization of the Aerobic and has the highest pairwise 16S rRNA gene sequence similarity with Sphingomonas glacialis (98.3%), Anoxygenic Phototrophic Bacterium Sphingomonas psychrolutea (96.8%), and Sphingomonas melonis (96.5%). -

Characterization of Stress Tolerance and Metabolic Capabilities of Acidophilic Iron-Sulfur-Transforming Bacteria and Their Relevance to Mars
Characterization of stress tolerance and metabolic capabilities of acidophilic iron-sulfur-transforming bacteria and their relevance to Mars Dissertation zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften – Dr. rer. nat. – vorgelegt von Anja Bauermeister aus Leipzig Im Fachbereich Chemie der Universität Duisburg-Essen 2012 Die vorliegende Arbeit wurde im Zeitraum von März 2009 bis Dezember 2012 im Arbeitskreis von Prof. Dr. Hans-Curt Flemming am Biofilm Centre (Fakultät für Chemie) der Universität Duisburg-Essen und in der Abteilung Strahlenbiologie (Institut für Luft- und Raumfahrtmedizin, Deutsches Zentrum für Luft- und Raumfahrt, Köln) durchgeführt. Tag der Einreichung: 07.12.2012 Tag der Disputation: 23.04.2013 Gutachter: Prof. Dr. H.-C. Flemming Prof. Dr. W. Sand Vorsitzender: Prof. Dr. C. Mayer Erklärung / Statement Hiermit versichere ich, dass ich die vorliegende Arbeit mit dem Titel „Characterization of stress tolerance and metabolic capabilities of acidophilic iron- sulfur-transforming bacteria and their relevance to Mars” selbst verfasst und keine außer den angegebenen Hilfsmitteln und Quellen benutzt habe, und dass die Arbeit in dieser oder ähnlicher Form noch bei keiner anderen Universität eingereicht wurde. Herewith I declare that this thesis is the result of my independent work. All sources and auxiliary materials used by me in this thesis are cited completely. Essen, 07.12.2012 Table of contents Abbreviations ....................................................................................... -

Experimental and Statistical Analysis of Nutritional Requirements for the Growth of the Extremophile Deinococcus Geothermalis DSM 11300
Experimental and statistical analysis of nutritional requirements for the growth of the extremophile Deinococcus geothermalis DSM 11300 Julie Bornot, Cesar Arturo Aceves-Lara, Carole Molina-Jouve, Jean-Louis Uribelarrea, Nathalie Gorret To cite this version: Julie Bornot, Cesar Arturo Aceves-Lara, Carole Molina-Jouve, Jean-Louis Uribelarrea, Nathalie Gor- ret. Experimental and statistical analysis of nutritional requirements for the growth of the ex- tremophile Deinococcus geothermalis DSM 11300. Extremophiles, Springer Verlag, 2014, 18 (6), pp.1009-1021. 10.1007/s00792-014-0671-8. hal-01268947 HAL Id: hal-01268947 https://hal.archives-ouvertes.fr/hal-01268947 Submitted on 11 Mar 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/22999 Official URL: https://doi.org/10.1007/s00792-014-0671-8 To cite this version: Bornot, Julie and Aceves-Lara, César-Arturo and Molina-Jouve, Carole and Uribelarrea, Jean-Louis and Gorret, Nathalie Experimental and statistical analysis of nutritional requirements for the growth of the extremophile Deinococcus geothermalis DSM 11300. -

Bimodal Emotion Recognition Model for Minnan Songs
information Article Bimodal Emotion Recognition Model for Minnan Songs Zhenglong Xiang 1 , Xialei Dong 1, Yuanxiang Li 1,2, Fei Yu 2,*, Xing Xu 2,3 and Hongrun Wu 2,* 1 School of Computer Science, Wuhan University, Wuhan 430072, China; [email protected] (Z.X.); [email protected] (X.D.); [email protected] (Y.L.) 2 School of Physics and Information Engineering, Minnan Normal University, Zhangzhou 363000, China; [email protected] 3 College of Information and Engineering, Jingdezhen Ceramic Institute, Jingdezhen 333000, China * Correspondence: [email protected] (F.Y.); [email protected] (H.W.) Received: 29 January 2020; Accepted: 2 March 2020; Published: 4 March 2020 Abstract: Most of the existing research papers study the emotion recognition of Minnan songs from the perspectives of music analysis theory and music appreciation. However, these investigations do not explore any possibility of carrying out an automatic emotion recognition of Minnan songs. In this paper, we propose a model that consists of four main modules to classify the emotion of Minnan songs by using the bimodal data—song lyrics and audio. In the proposed model, an attention-based Long Short-Term Memory (LSTM) neural network is applied to extract lyrical features, and a Convolutional Neural Network (CNN) is used to extract the audio features from the spectrum. Then, two kinds of extracted features are concatenated by multimodal compact bilinear pooling, and finally, the concatenated features are input to the classifying module to determine the song emotion. We designed three experiment groups to investigate the classifying performance of combinations of the four main parts, the comparisons of proposed model with the current approaches and the influence of a few key parameters on the performance of emotion recognition. -

BOSS on EXPOSE-R2-Comparative Investigations on Biofilm and Planktonic Cells of Deinococcus Geothermalis As Mission Preparation Tests
EPSC Abstracts Vol. 8, EPSC2013-930, 2013 European Planetary Science Congress 2013 EEuropeaPn PlanetarSy Science CCongress c Author(s) 2013 BOSS on EXPOSE-R2-Comparative Investigations on Biofilm and Planktonic cells of Deinococcus geothermalis as Mission Preparation Tests Corinna Panitz1,2, Petra Rettberg2, Jan Frösler3, Prof. H. - C. Flemming3, Elke Rabbow2, Günther Reitz2 1RWTH Aachen/Medical Faculty, Inst.of Aerospace Medicine - Aachen, Germany, 2DLR, Inst.of Aerospace Medicine, Radiation Biology Dep. - Köln, Germany,3Universität Duisburg-Essen, Fakultät für Chemie - Biofilm Centre - Essen, Germany1; ([email protected] / Fax: +49-2203-61970) Abstract not only bacteria and Archaea, have mechanisms by which they can adhere to surfaces and to each other. Biofilms are of interest for Astrobiological investi- Biofilms are characterized by structural heterogeneity, gations since they are one of the oldest clear signs of genetic diversity, and complex community inter- life on Earth. In the experiment BOSS the hypothesis actions using the EPS matrix for stable, long-term interactions and as an external digestion system. This will be tested if the biofilm form of life with micro- matrix is strong enough that under certain conditions organisms embedded and aggregated in their EPS biofilms can even become fossilized. Usually, abiotic matrix is suited to support long-term survival of particles are associated with the “sticky” biofilm microorganisms under the harsh environmental con- matrix. One benefit of this environment is increased ditions as they exist in space and on Mars and is resistance to different chemical and physical agents superior to the same bacteria in the form of plank- as the dense extracellular matrix and the outer layer tonic cultures. -

The Human Microbiome and Its Link in Prostate Cancer Risk and Pathogenesis Paul Katongole1,2* , Obondo J
Katongole et al. Infectious Agents and Cancer (2020) 15:53 https://doi.org/10.1186/s13027-020-00319-2 REVIEW Open Access The human microbiome and its link in prostate cancer risk and pathogenesis Paul Katongole1,2* , Obondo J. Sande3, Moses Joloba3, Steven J. Reynolds4 and Nixon Niyonzima5 Abstract There is growing evidence of the microbiome’s role in human health and disease since the human microbiome project. The microbiome plays a vital role in influencing cancer risk and pathogenesis. Several studies indicate microbial pathogens to account for over 15–20% of all cancers. Furthermore, the interaction of the microbiota, especially the gut microbiota in influencing response to chemotherapy, immunotherapy, and radiotherapy remains an area of active research. Certain microbial species have been linked to the improved clinical outcome when on different cancer therapies. The recent discovery of the urinary microbiome has enabled the study to understand its connection to genitourinary malignancies, especially prostate cancer. Prostate cancer is the second most common cancer in males worldwide. Therefore research into understanding the factors and mechanisms associated with prostate cancer etiology, pathogenesis, and disease progression is of utmost importance. In this review, we explore the current literature concerning the link between the gut and urinary microbiome and prostate cancer risk and pathogenesis. Keywords: Prostate cancer, Microbiota, Microbiome, Gut microbiome, And urinary microbiome Introduction by which the microbiota can alter cancer risk and pro- The human microbiota plays a vital role in many life gression are primarily attributed to immune system processes, both in health and disease [1, 2]. The micro- modulation through mediators of chronic inflammation. -

Access to Electronic Thesis
Access to Electronic Thesis Author: Khalid Salim Al-Abri Thesis title: USE OF MOLECULAR APPROACHES TO STUDY THE OCCURRENCE OF EXTREMOPHILES AND EXTREMODURES IN NON-EXTREME ENVIRONMENTS Qualification: PhD This electronic thesis is protected by the Copyright, Designs and Patents Act 1988. No reproduction is permitted without consent of the author. It is also protected by the Creative Commons Licence allowing Attributions-Non-commercial-No derivatives. If this electronic thesis has been edited by the author it will be indicated as such on the title page and in the text. USE OF MOLECULAR APPROACHES TO STUDY THE OCCURRENCE OF EXTREMOPHILES AND EXTREMODURES IN NON-EXTREME ENVIRONMENTS By Khalid Salim Al-Abri Msc., University of Sultan Qaboos, Muscat, Oman Mphil, University of Sheffield, England Thesis submitted in partial fulfillment for the requirements of the Degree of Doctor of Philosophy in the Department of Molecular Biology and Biotechnology, University of Sheffield, England 2011 Introductory Pages I DEDICATION To the memory of my father, loving mother, wife “Muneera” and son “Anas”, brothers and sisters. Introductory Pages II ACKNOWLEDGEMENTS Above all, I thank Allah for helping me in completing this project. I wish to express my thanks to my supervisor Professor Milton Wainwright, for his guidance, supervision, support, understanding and help in this project. In addition, he also stood beside me in all difficulties that faced me during study. My thanks are due to Dr. D. J. Gilmour for his co-supervision, technical assistance, his time and understanding that made some of my laboratory work easier. In the Ministry of Regional Municipalities and Water Resources, I am particularly grateful to Engineer Said Al Alawi, Director General of Health Control, for allowing me to carry out my PhD study at the University of Sheffield. -

Sphingomonas Sp. Cra20 Increases Plant Growth Rate and Alters Rhizosphere Microbial Community Structure of Arabidopsis Thaliana Under Drought Stress
fmicb-10-01221 June 4, 2019 Time: 15:3 # 1 ORIGINAL RESEARCH published: 05 June 2019 doi: 10.3389/fmicb.2019.01221 Sphingomonas sp. Cra20 Increases Plant Growth Rate and Alters Rhizosphere Microbial Community Structure of Arabidopsis thaliana Under Drought Stress Yang Luo1, Fang Wang1, Yaolong Huang1, Meng Zhou1, Jiangli Gao1, Taozhe Yan1, Hongmei Sheng1* and Lizhe An1,2* 1 Ministry of Education Key Laboratory of Cell Activities and Stress Adaptations, School of Life Sciences, Lanzhou University, Lanzhou, China, 2 The College of Forestry, Beijing Forestry University, Beijing, China The rhizosphere is colonized by a mass of microbes, including bacteria capable of Edited by: promoting plant growth that carry out complex interactions. Here, by using a sterile Camille Eichelberger Granada, experimental system, we demonstrate that Sphingomonas sp. Cra20 promotes the University of Taquari Valley, Brazil growth of Arabidopsis thaliana by driving developmental plasticity in the roots, thus Reviewed by: Muhammad Saleem, stimulating the growth of lateral roots and root hairs. By investigating the growth Alabama State University, dynamics of A. thaliana in soil with different water-content, we demonstrate that Cra20 United States Andrew Gloss, increases the growth rate of plants, but does not change the time of reproductive The University of Chicago, transition under well-water condition. The results further show that the application of United States Cra20 changes the rhizosphere indigenous bacterial community, which may be due *Correspondence: to the change in root structure. Our findings provide new insights into the complex Hongmei Sheng [email protected] mechanisms of plant and bacterial interactions. The ability to promote the growth of Lizhe An plants under water-deficit can contribute to the development of sustainable agriculture. -

Download Article
Advances in Social Science, Education and Humanities Research, volume 205 The 2nd International Conference on Culture, Education and Economic Development of Modern Society (ICCESE 2018) The Research on Docking Model of Rural Tourism across the Taiwan Straits Xicong Zheng Tourism Management Minnan Normal University Zhangzhou, China 363000 Abstract—In recent years, rural travel has become a new tourism promotion, and the most basic form of rural tourism form of tourism in the industry of China. It is a tourism product should have: developed by the combination of rural and agricultural production and the resource advantages of the countryside, so 1. Located in a rural area. that the rural industrial economy will show new vitality and 2. Village function: It consists of small businesses, open realize the sustainable development of rural tourism. This paper spaces, natural environments, monuments, traditional societies analyzes and compares the mode of rural tourism by exploring and customs. docking pattern on both sides of the Taiwan Straits, and puts forward some suggestions. 3. Village size: Buildings and the environment are small scales. Keywords—both sides of the Taiwan Straits; rural tourism; docking pattern 4. With traditional qualities, slow growth rate, and close relationship with local families. I. INTRODUCTION 5. The combination of rural environment, economy, history Rural tourism originated in France in 1855, and has a and location. history of more than 100 years. In the 1960s, rural tourism in The European Union and the World Organization for the modern sense began in Spain, and then it developed rapidly Economic Cooperation and Development (1994) defined rural in developed countries. -
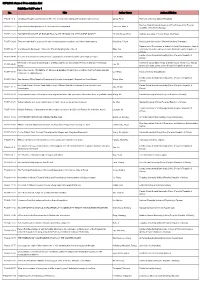
ICP2016: Cancel Presentation List
ICP2016: Cancel Presentation List Exhibition Hall Poster 1 No. Title Author Name Author Affiliation PS25P-01-6 Creativity through social interaction: The role of perspective taking and interpersonal closeness Oztop, Pinar Plymouth University (United Kingdom) Moscow State University, Institute of Psychology of the Russian PS25P-01-12 Signs of intellectual giftedness: the resource-based approach Trifonova, Alina V Academy of Sciences (Russia) PS25P-01-28 FACTOR STRUCTURE OF BAR-ON EQ-i YOUTH VERSON IN A VIETNAMESE SAMPLE Thi Mai Huong, Phan Vietnam Academy of Social Scices (Viet Nam) PS25P-04-29 The personal belief in a just world, school related justice cognitions, and school achievement Kiral Ucar, Gözde Martin Luther University of Halle-Wittenberg (Germany) Department of Psychology at School of Social Development, Central PS25P-04-37 Going Beyond the Beauty - Trust Link: The Moderating Role of mood Zhao, Na University of Finance and Economics (China (People's Republic of China)) Central China Normal University (China (People's Republic of PS25P-04-44 Need for Self-uniqueness Influences the Evaluations of Gender Counter-stereotypical People Tan, Kaihua China)) Research on Disputes between Labor and Management by Using Game Theory in China's Professional College of Sports Engineering and Information Technology, Wuhan PS25P-04-45 Luo, Qi Sports Sports University, China (China (People's Republic of China)) PSYCHOLOGICAL FEATURES OF DECISION-MAKING POLICY IN A CONFLICT SITUATIONS AMONG PS25P-07-48 Li, Yelena Turan University (Kazakhstan) UNIVERSITY -

Participants: (In Order of the Surname)
Participants 31 Participants: (in order of the surname) Yansong Bai yyyòòòttt: Jilin University, Changchun. E-mail: [email protected] Jianhai Bao ïïï°°°: Central South University, Changsha. E-mail: [email protected] Chuanzhong Chen •••DDD¨¨¨: Hainan Normal University, Haikou. E-mail: [email protected] Dayue Chen •••ŒŒŒ: Peking University, Beijing. E-mail: [email protected] Haotian Chen •••hhhUUU: Jilin University, Changchun. E-mail: [email protected] Longyu Chen •••999ˆˆˆ: Peking University, Beijing. E-mail: [email protected] Man Chen •••ùùù: Capital Normal University, Beijing. E-mail: [email protected] Mu-Fa Chen •••777{{{: Beijing Normal University, Beijing. E-mail: [email protected] Shukai Chen •••ÓÓÓppp: Beijing Normal University, Beijing. E-mail: [email protected] Xia Chen •••ggg: Jilin University, Changchun; University of Tennessee, USA. E-mail: [email protected] Xin Chen •••lll: Shanghai Jiao Tong University, Shanghai. E-mail: [email protected] Xue Chen •••ÆÆÆ: Capital Normal University, Beijing. E-mail: [email protected] Zengjing Chen •••OOO¹¹¹: Shandong University, Jinan. E-mail: [email protected] 32 Participants Huihui Cheng §§§¦¦¦¦¦¦: North China University of Water Resources and Electric Power, Zhengzhou E-mail: [email protected] Lan Cheng §§§===: Central South University, Changsha. E-mail: [email protected] Zhiwen Cheng §§§“““>>>: Beijing Normal University, Beijing. E-mail: [email protected] Michael Choi éééRRRZZZ: The Chinese University of Hong Kong, Shenzhen. E-mail: [email protected] Bowen Deng """ÆÆÆ©©©: Jilin University, Changchun. E-mail: [email protected] Changsong Deng """ttt: Wuhan University, Wuhan. E-mail: [email protected] Xue Ding ¶¶¶ÈÈÈ: Jilin University, Changchun. -

Microbiology in Shale: Alternatives for Enhanced Gas Recovery
Graduate Theses, Dissertations, and Problem Reports 2015 Microbiology in Shale: Alternatives for Enhanced Gas Recovery Yael Tarlovsky Tucker Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Tucker, Yael Tarlovsky, "Microbiology in Shale: Alternatives for Enhanced Gas Recovery" (2015). Graduate Theses, Dissertations, and Problem Reports. 6834. https://researchrepository.wvu.edu/etd/6834 This Dissertation is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Dissertation in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Dissertation has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. Microbiology in Shale: Alternatives for Enhanced Gas Recovery Yael Tarlovsky Tucker Dissertation submitted to the Davis College of Agriculture, Natural Resources and Design at West Virginia University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Genetics and Developmental Biology Jianbo Yao, Ph.D., Chair James Kotcon, Ph.D.