Morphological and Ecological Studies of the Mexican Bean Beetle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Twenty-Five Pests You Don't Want in Your Garden
Twenty-five Pests You Don’t Want in Your Garden Prepared by the PA IPM Program J. Kenneth Long, Jr. PA IPM Program Assistant (717) 772-5227 [email protected] Pest Pest Sheet Aphid 1 Asparagus Beetle 2 Bean Leaf Beetle 3 Cabbage Looper 4 Cabbage Maggot 5 Colorado Potato Beetle 6 Corn Earworm (Tomato Fruitworm) 7 Cutworm 8 Diamondback Moth 9 European Corn Borer 10 Flea Beetle 11 Imported Cabbageworm 12 Japanese Beetle 13 Mexican Bean Beetle 14 Northern Corn Rootworm 15 Potato Leafhopper 16 Slug 17 Spotted Cucumber Beetle (Southern Corn Rootworm) 18 Squash Bug 19 Squash Vine Borer 20 Stink Bug 21 Striped Cucumber Beetle 22 Tarnished Plant Bug 23 Tomato Hornworm 24 Wireworm 25 PA IPM Program Pest Sheet 1 Aphids Many species (Homoptera: Aphididae) (Origin: Native) Insect Description: 1 Adults: About /8” long; soft-bodied; light to dark green; may be winged or wingless. Cornicles, paired tubular structures on abdomen, are helpful in identification. Nymph: Daughters are born alive contain- ing partly formed daughters inside their bodies. (See life history below). Soybean Aphids Eggs: Laid in protected places only near the end of the growing season. Primary Host: Many vegetable crops. Life History: Females lay eggs near the end Damage: Adults and immatures suck sap from of the growing season in protected places on plants, reducing vigor and growth of plant. host plants. In spring, plump “stem Produce “honeydew” (sticky liquid) on which a mothers” emerge from these eggs, and give black fungus can grow. live birth to daughters, and theygive birth Management: Hide under leaves. -

Florida Predatory Stink Bug (Unofficial Common Name), Euthyrhynchus Floridanus(Linnaeus) (Insecta: Hemiptera: Pentatomidae)1 Frank W
EENY157 Florida Predatory Stink Bug (unofficial common name), Euthyrhynchus floridanus (Linnaeus) (Insecta: Hemiptera: Pentatomidae)1 Frank W. Mead and David B. Richman2 Introduction Distribution The predatory stink bug, Euthyrhynchus floridanus (Lin- Euthyrhynchus floridanus is primarily a Neotropical species naeus) (Figure 1), is considered a beneficial insect because that ranges within the southeastern quarter of the United most of its prey consists of plant-damaging bugs, beetles, States. and caterpillars. It seldom plays a major role in the natural control of insects in Florida, but its prey includes a number Description of economically important species. Adults The length of males is approximately 12 mm, with a head width of 2.3 mm and a humeral width of 6.4 mm. The length of females is 12 to 17 mm, with a head width of 2.4 mm and a humeral width of 7.2 mm. Euthyrhynchus floridanus (Figure 2) normally can be distinguished from all other stink bugs in the southeastern United States by a red- dish spot at each corner of the scutellum outlined against a blue-black to purplish-brown ground color. Variations occur that might cause confusion with somewhat similar stink bugs in several genera, such as Stiretrus, Oplomus, and Perillus, but these other bugs have obtuse humeri, or at least lack the distinct humeral spine that is present in adults of Euthyrhynchus. In addition, species of these genera Figure 1. Adult of the Florida predatory stink bug, Euthyrhynchus known to occur in Florida have a short spine or tubercle floridanus (L.), feeding on a beetle. situated on the lower surface of the front femur behind the Credits: Lyle J. -

Mexican Bean Beetle (Suggested Common Name), Epilachna Varivestis Mulsant (Insecta: Coleoptera: Coccinellidae)1 H
EENY-015 Mexican Bean Beetle (suggested common name), Epilachna varivestis Mulsant (Insecta: Coleoptera: Coccinellidae)1 H. Sanchez-Arroyo2 Introduction apparently eradicated from Florida in 1933, but was found again in 1938 and by 1942 was firmly established. The family Coccinellidae, or ladybird beetles, is in the order Coleoptera. This family is very important economically because it includes some highly beneficial insects as well as Description two serious pests: the squash lady beetle, Epilachna borealis Eggs Fabricius, and the Mexican bean beetle, Epilachna varivestis Eggs are approximately 1.3 mm in length and 0.6 mm in Mulsant. width, and are pale yellow to orange-yellow in color. They are typically found in clusters of 40 to 75 on the undersides The Mexican bean beetle has a complete metamorphosis of bean leaves. with distinct egg, larval, pupal, and adult stages. Unlike most of the Coccinellidae, which are carnivorous and feed upon aphids, scales, and other small insects, this species attacks plants. Distribution The Mexican bean beetle is believed to be native to the plateau region of southern Mexico. This insect is found in the United States (in most states east of the Rocky Moun- tains) and Mexico. In eastern regions, the pest is present wherever beans are grown, while western infestations are in isolated areas, depending upon the local environment and precipitation. The insect is not a serious pest in Guatemala and Mexico, but is very abundant in several areas in the western United States. The southern limit of the known distribution is in Guatemala and the northern limit is Figure 1. -

Spined Soldier Bug
Beneficial Species Profile Photo credit: Russ Ottens, University of Georgia, Bugwood.org; Gerald J. Lenhard, Louisiana State University, Bugwood.org Common Name: Spined soldier bug Scientific Name: Podisus maculiventris Order and Family: Hemiptera; Pentatomidae Size and Appearance: Length (mm) Appearance Egg 1 mm Around the operculum (lid/covering) on the egg there are long projections; eggs laid in numbers of 17 -70 in oval masses; cream to black in color. Larva/Nymph 1.3 – 10 mm 1st and 2nd instars: black head and thorax; reddish abdomen; black dorsal and lateral plates. Younger instars are gregarious; cannibalistic. 3rd instar: black head and thorax; reddish abdomen with black, orange, and white bar-shaped and lateral markings 4th instar: similar to 3rd instar; wing pads noticeable 5th instar: prominent wing pads; mottled brown head and thorax; white or tan and black markings on abdomen. Adult 11 mm Spine on each shoulder; mottled brown body color; females larger than males; 2 blackish dots at the 3rd apical (tip) of each hind femur; 1-3 generations a year. Pupa (if applicable) Type of feeder (Chewing, sucking, etc.): Nymph and adult: Piercing-sucking Host/s: Spined soldier bugs are predators and feed on many species of insects including the larval stage of beetles and moths. These insects are found on many different crops including alfalfa, soybeans, and fruit. Some important pest insect larvae prey include Mexican bean beetle larvae, European corn borer, imported cabbageworm, fall armyworm, corn earworm, and Colorado potato beetle larvae. If there is not enough prey, the spined soldier bug may feed on plant juices, but this does not damage the plant. -

Organic Management of Mexican Bean Beetle (Epilachna Varivestis Mulsant) in Snap Bean (Phaseolus Vulgaris L.)
Graduate Theses, Dissertations, and Problem Reports 2008 Organic management of Mexican bean beetle (Epilachna varivestis Mulsant) in snap bean (Phaseolus vulgaris L.) Tiffany L. Fess West Virginia University Follow this and additional works at: https://researchrepository.wvu.edu/etd Recommended Citation Fess, Tiffany L., "Organic management of Mexican bean beetle (Epilachna varivestis Mulsant) in snap bean (Phaseolus vulgaris L.)" (2008). Graduate Theses, Dissertations, and Problem Reports. 2607. https://researchrepository.wvu.edu/etd/2607 This Thesis is protected by copyright and/or related rights. It has been brought to you by the The Research Repository @ WVU with permission from the rights-holder(s). You are free to use this Thesis in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you must obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/ or on the work itself. This Thesis has been accepted for inclusion in WVU Graduate Theses, Dissertations, and Problem Reports collection by an authorized administrator of The Research Repository @ WVU. For more information, please contact [email protected]. Organic Management of Mexican Bean Beetle (Epilachna varivestis Mulsant) in Snap Bean (Phaseolus vulgaris L.) Tiffany L. Fess Thesis submitted to the Davis College of Agriculture, Forestry, and Consumer Sciences at West Virginia University in partial fulfillment of the requirements for the degree of Master of Science in Horticulture Sven Verlinden, Ph.D., Major Professor Linda Butler, Ph.D James Kotcon, Ph.D Department of Plant and Soil Sciences Morgantown, West Virginia 2008 Keywords: Green snap beans, Mexican Bean Beetle, Pediobius foveolatus, Organic production, Row cover, Planting date Abstract Organic Management of Mexican Bean Beetle (Epilachna varivestis Mulsant) Infestations in Snap Bean (Phaseolus vulgaris L.) Crops Tiffany L. -

Nottingham L D 2017.Pdf
Development and Evaluation of Integrated Approaches for Managing of Mexican Bean Beetle, Epilachna varivestis Mulsant Louis Bove Nottingham Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy In Entomology Thomas P. Kuhar, Chair Ramón A. Arancibia D. Ames Herbert Timothy J. Kring Peter B. Schultz December 5, 2016 Blacksburg, VA Keywords: Integrated pest management (IPM), Mexican bean beetle, Epilachna varivestis, Phaseolus, snap bean, lima bean, agroecosystem, cultivars, susceptible, cultural management, seed-treatment, thiamethoxam, natural enemies, Podisus maculiventris Development and Evaluation of Integrated Approaches for Managing of Mexican Bean Beetle, Epilachna varivestis Mulsant Louis Bove Nottingham ABSTRACT The Mexican bean beetle, Epilachna varivestis Mulsant, is a major pest of snap beans, Phaseolus vulgaris L. in the Central Appalachian region of the United States. To develop pertinent research objectives, background information on this pest was gathered from literature sources and personal communications with growers, extension agents and other agricultural professionals. In objective one, Mexican bean beetle preference, developmental success and plant injury were compared among three snap bean and three lima bean cultivars in field and greenhouse trials. The cultivar ‘Dragon’s Tongue’ was the most preferred, suitable for development, and prone to injury. Growers may benefit from growing less susceptible cultivars, or by using ‘Dragon’s Tongue’ in trap cropping or push-pull strategies. In objective two, Mexican bean beetle densities, feeding injury, and yield were compared among snap beans grown on metallized plastic (highly reflective), white plastic, black plastic, and bare soil. Metallized plastic provided the greatest level of control, and resulted in the highest yields. -

Mexican Bean Beetle.Pub
CORNELL COOPERATIVE EXTENSION OF ONEIDA COUNTY 121 Second Street Oriskany, NY 13424-9799 (315) 736-3394 or (315) 337-2531 FAX: (315) 736-2580 MEXICAN BEAN BEETLE (Epilachna varivestis) Larvae and associated damage Adult with summer colora- on bean. Pupa and associated larval dam- tion on bean. ©The Bug Network, www. age on bean. ©The Bug Network, www. forestryimages.org Photo ©The Bug Network, www. forestryimages.org Photo courtesy of Whitney Cran- forestryimages.org Photo cour- courtesy of Whitney Cran- shaw, Colorado State Univer- tesy of Whitney Cranshaw, shaw, Colorado State Uni- sity Colorado State University versity Injury The Mexican bean beetle, formerly called the bean ladybird, is one of the most destructive insect pests of beans in New York State. The beetle feeds on the leaves of almost all types of beans including snap, lima, pinto, navy, kid- ney and soybeans. With snap beans, bush varieties seem to be attacked more readily than pole varieties. Most of the damage from the Mexican bean beetle occurs during July and August. Both the adult and the larval stages feed on the foliage chewing out holes in the leaves. They usually feed on the undersides of the leaves, and sometimes will attack young pods and stems. As a result of feeding, only the veins are left giving the leaves a lacy appearance. Yield may be greatly reduced and the entire planting may be destroyed in severe infestations. Description The Mexican bean beetle is a convex beetle, about 1/3 inch long, and pale yellow to copper in color with 16 black spots on its back. -
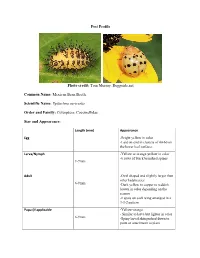
Pest Profile Photo Credit: Tom Murray, Bugguide.Net
Pest Profile Photo credit: Tom Murray, Bugguide.net Common Name: Mexican Bean Beetle Scientific Name: Epilachna varivestis Order and Family: Coleoptera: Coccinellidae Size and Appearance: Length (mm) Appearance Egg -Bright yellow in color -Laid on-end in clusters of 40-60 on the lower leaf surfaces Larva/Nymph -Yellow or orange-yellow in color -6 rows of black branched spines 1-9mm Adult -Oval shaped and slightly larger than other ladybeetles 6-9mm -Dark yellow to copper to reddish brown in color depending on the season -8 spots on each wing arranged in a 3-3-2 pattern Pupa (if applicable -Yellow-orange - Similar to larva but lighter in color 6-9mm -Spiny larval skin pushed down to point of attachment to plant Type of feeder (Chewing, sucking, etc.): Chewing Host plant/s: The Mexican bean beetle can be found in gardens and bean fields on lima beans, snap beans, clover, and alfalfa. Occasionally the beetles will feed on soybeans and cowpeas. Description of Damage (larvae and adults): The Mexican bean beetle larvae and adults feed on the underside of the leaves between the veins, leaving behind a lacy skeleton appearance as the leaves die. The adult beetles will feed on the leaves until they become unappealing, then they will eat the blooms, stem, and green bean pods. References: Boone, M., Moisset, B., McLeon, R., Entz, C., Quinn, M., Moyer, T., & Kropiewnicki, T. (2004, November 23). Species Epilachna varivestis – Mexican Bean Beetle. Retrieved January 15, 2016, from http://bugguide.net/node/view/8712 Cranshaw, W. (2004). Garden insects of North America: The ultimate guide to backyard bugs. -

The Mexican Bean Beetle in Connecticut
Bulletin 332 December, 1931 THE. MEXICAN BEAN BEETLE IN CONNECTICUT ROGER B. FRIEND and NEELY TURNER CONTROLLING THE BEAN BEETLE Hean heetle injury may I,? avoided by sprayi~~gor dusting com- I,ine<l \vitli certain cultural practices. Spr:iying is more effective. 'Therr are two generatinns of the illsect a year and 110th larvae and adtilt.; feed on the plants. Spray Magnesium arsctiate .... 3 lhs. Casein lime ....... 2 lbs. Water .....100 gals. Dust Magnesirini ai-srnatc .... 111). flydrated lime ...... 5 11>s. or l3:irinm flo<bsilicate ..... 1 111. IIydmtrd lime ........ 5 Ills. To Apply Insecticide 1. Hl~plyahnut June 15 and Ji~ne25 to ?a~-Iyplantings. For later plantinjis, ahnl~tJi1520, JII~T30. atid August 0. Limn and pole healls may rnll1il.e :it1 lire ap]ilic:ltir,ns. 2. Alqily to the under sirlc of leaves. 3. Cover the entire scirface. 4. Spray or dust before the injury is severe. Afterwards it is too late. 5. The iiisecticidcs ~nentionctlalluue shoulrl not be used aftrr the pods are lialf-grown unless the l~eansare waslle(l before mar- lieting. A pyrethrum-soap mixture may be used instead. Cultural Practices 1. Destroy the hibernating quarters of the adults near cultivated fields. 2. Plow under or pull up and destroy all beans as soon as the , crop is harvested. 3. The shorter the growing period, the less time the plants are exposed to attack. Pro~nioterapid growth and early maturity by thin planting, proper fertiliration, and thorough cultivation. THE MEXICAN BEAN BEETLE IN CONNECTICUT History and distriht~tion ...... 73 Culti~mlcontrol ........... -

Insect Identification Laboratory Annual Report 2013
ID Lab Publication Revised 2013 Insect Identification Laboratory Annual Report 2013 Eric R. Day Douglas G. Pfeiffer Theresa Dellinger Olivia McCraw Department of Entomology College of Agriculture and Life Sciences Virginia Cooperative Extension Virginia Polytechnic Institute and State University TABLE OF CONTENTS Topic Page Introduction ......................................................................................................................... 2 Collaborators ....................................................................................................................... 3 Total Number of Specimens Received ................................................................................ 4 Specimens Received by Month ........................................................................................... 5 Arthropods Received by Host Plants ................................................................................ 31 Source of Insects by County ............................................................................................. 41 INTRODUCTION A total of 954 requests were received in 2013. This report summarizes the activity of the Insect Identification Laboratory at Virginia Tech for 2013. The laboratory is located in 205A Price Hall. Eric Day, Lab Manager, and Doug Pfeiffer, Extension Entomologist, Department of Entomology manage the lab. Charlotte Oliver, Tree Dellinger, and Olivia McCraw also worked in the Lab in 2013. Of the samples received in 2013, 79% of the samples, 749 out of 954, requested -

U. S. Departme Agriculture [Exican Bean Beetle T
U. S. DEPARTME AGRICULTURE FARMERS' BULLETIN No. 1407 !i i [EXICAN BEAN BEETLE T HE Mexican bean beetle in its range of distribu T tion is the most serious insect enemy of beans in the United States. In the Southeast it was first found in Alabama in 1920, and now infests large areas in Alabama, Georgia, Tennessee, and Ken tucky, and smaller areas in the Carolinas, Virginia, Mississippi, and Ohio. It is spreading northward trapidly and may soon extend its range over most of the Eastern States. The adult is a copper-colored beetle, bearing eight black spots on e^ch wing cover, and is about one- ifourth of an inch long. The larva is orange colored and is frequently described as " fuzzy." This insect feeds on the plants of all kinds of table beans, cowpeas, soy beans, beggarweed, and others of lesser importance. The principal injury is done to the foliage, but in cases of heavy infestation green pods also are destroyed. Magnesium arsenate, used as a spray or as a dust, is the most promising insecticide. Calcium arsenate combined with hydrated lime is also useful. Heavily infested fields should be plowed under as soon as the crop is off, and the grower should not plant more beans than can be properly treated. Washington, D. G. May, 1924 THE MEXICAN BEAN BEETLE' IN THE EAST. By NEALE F. HOWARD, Entomologist, Truck-Crop Insect Investigations, Bureau of Entomology. CONTENTS. Page. Fa«e. The most destructive insect to growing Natural agencies of control 7 bi-aiis 1 I'ontrol measures 8 Appearance of insect and nature of Sprays 8 dauiaso 1 Directions for applying the spray. -

The Mexican Bean Beetle
'-----_.- / BULLETIN NO. 176 ,z.... JANUARY, 1931 UNIVERSITY OF WYOMING AGRICUL TURAL EXPERIMENT STATION The Mexican Bean Beetle Bulletins will be sent free upon request. Address: Director of Experiment Station, Laramie, Wyoming. UNIVERSITY OF WYOMING Agricultural Experiment Station LARAMIE, WYOMING BOARD OF TRUSTEES Officer. WILL M. LyNN · President JOJ;EPH A. ELLIOTT Vice President FHI':O W. (;EDDES Treasurer FA Y E. J;AllTH , Secretary Ie. D. Ji'ULLER Ftscal Agent Executive Committee WILL M. LYNN ~'RANK A. HOLLlDAY JOSEPH A. ELLIOTT WALLACE C. BOKI> Appoin ted Members Term Expires 1921 ; JOSEPH A. ELLIOTT 1933 1921. FRED W. WWOES 1933 1923 FRANK ALAN HOLLIDAY 1933 1925 HARRIETT T. GRIEVE 1931 1925 J. M. SCHWOOB 1931 1927 WILL M. LYNN 1933 192Q WALLACE C. BOND 1935 1929 MABELLE G. OVlATT 1935 FHAXl\. C. E~J1~H~U~, Governor of \V)·omillg .....................•.•..••.......... Ex Ofticio I\ATIIA HI"E A. MOHTO", State Superintendent of Public Instruction Ex Ontclo A. G. CHANE, Ph.D., President of the Uutveraity ..............• , ...........••..... Ex Offtciu STATION STAFF A. G. CIlA"E, I'h.D Preaideut J. A. HILL, B.S Wool Specialist. IJirettor FAY E. SMITH . Secret,"·y M. A. ALEXANDEH, M.S Assistant Animal Husbandman o. A. DEATH, M.A. ..............•..................... Sta tiun Chemtst "ROBERT II. BURNS, M.S................ .. Assistant Wool Specinl ist. C. L. COR.KINS, M.S Research Assoctate Entomologist and Apiculturist T. J. UU~~E'VALIJ. Ai.S......... Asst-a.uu soil lnvest iun tious *tJ. E. ECKERT, M.S Associate Apiculturist U. S. Bee Culture Field Station CECIL ELl>b:H, D.V.M., !l.S : Resen rch [,,,tholo;:ist *II.