Enhanced Frugivory on Invasive Silene Latifolia in Its Native Range Due to Increased Oviposition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identification of White Campion (Silene Latifolia) Guaiacol O-Methyltransferase Involved in the Biosynthesis of Veratrole, a Key Volatile for Pollinator Attraction
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2012 Identification of white campion (Silene latifolia) guaiacol O-methyltransferase involved in the biosynthesis of veratrole, a key volatile for pollinator attraction Gupta, Alok K ; Akhtar, Tariq A ; Widmer, Alex ; Pichersky, Eran ; Schiestl, Florian P Abstract: BACKGROUND: Silene latifolia and its pollinator, the noctuid moth Hadena bicruris, repre- sent an open nursery pollination system wherein floral volatiles, especially veratrole (1, 2-dimethoxybenzene), lilac aldehydes, and phenylacetaldehyde are of key importance for floral signaling. Despite the important role of floral scent in ensuring reproductive success in S. latifolia, the molecular basis of scent biosyn- thesis in this species has not yet been investigated. RESULTS: We isolated two full-length cDNAs from S. latifolia that show similarity to rose orcinol O-methyltransferase. Biochemical analysis showed that both S. latifolia guaiacol O-methyltransferase1 (SlGOMT1) S. latifolia guaiacol O-methyltransferase2 (SlGOMT2) encode proteins that catalyze the methylation of guaiacol to form veratrole. A large Km value difference between SlGOMT1 ( 10 M) and SlGOMT2 ( 501 M) resulted that SlGOMT1 is31-fold more catalytically efficient than SlGOMT2. qRT-PCR expression analysis showed that theSlGOMT genes are specifically expressed in flowers and male S. latifolia flowers had 3- to 4-folds higher levelof GOMT gene transcripts than female flower tissues. Two related cDNAs, S. dioica O-methyltransferase1 (SdOMT1) and S. dioica O-methyltransferase2 (SdOMT2), were also obtained from the sister species Silene dioica, but the proteins they encode did not methylate guaiacol, consistent with the lack of ver- atrole emission in the flowers of this species. -

Local Adaptation for Life-History Traits in Silene Latifolia
Georgia Southern University Digital Commons@Georgia Southern Electronic Theses and Dissertations Graduate Studies, Jack N. Averitt College of Spring 2006 Local Adaptation for Life-History Traits in Silene Latifolia Brandy May Penna Follow this and additional works at: https://digitalcommons.georgiasouthern.edu/etd Recommended Citation Penna, Brandy May, "Local Adaptation for Life-History Traits in Silene Latifolia" (2006). Electronic Theses and Dissertations. 734. https://digitalcommons.georgiasouthern.edu/etd/734 This thesis (open access) is brought to you for free and open access by the Graduate Studies, Jack N. Averitt College of at Digital Commons@Georgia Southern. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons@Georgia Southern. For more information, please contact [email protected]. LOCAL ADAPTATION FOR LIFE-HISTORY TRAITS IN SILENE LATIFOLIA by BRANDY M. PENNA (Under the Direction of Lorne M. Wolfe) ABSTRACT A fundamental question in evolutionary ecology is how species adjust post colonization. The plant Silene latifolia was introduced to North America (NA) from Europe (EU) in the 1800s. The goal of this thesis was to test if Silene latifolia has become locally adapted across its range. My first experiment tested local adaptation of germination success to three temperatures across three latitudinal regions in a growth chamber using seeds from nine EU and NA populations. Germination success or speed was similar among latitudinal regions across continents. My second experiment examined local adaptation at a continental scale; I grew plants from 15 EU and NA populations in four common gardens across continents. Growth and survival for the first year revealed that plants grew larger in their respective continents. -

Section Elisanthe)
POPULATION GENETICS AND SPECIATION IN THE PLANT GENUS SILENE (SECTION ELISANTHE) by ANDREA LOUISE HARPER A thesis submitted to The University of Birmingham for the degree of DOCTOR OF PHILOSOPHY School of Biosciences The University of Birmingham 2009 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. ABSTRACT This thesis is concerned with speciation and population genetics in the plant genus Silene (section Elisanthe). The introductory chapter is a literature review covering characteristics of the species studied, and the current literature on their evolutionary dynamics and population genetics. The second and third chapters cover techniques used in all experiments, such as DNA extraction, sequencing and genotyping protocols, and explain the rationale behind the initial experimental design. The fourth chapter focuses on the multi-locus analysis of autosomal gene sequences from S. latifolia and S. dioica. The relationship between the two species was investigated using various analyses such as isolation modeling and admixture analysis providing estimates of evolutionary distance and extent of historical gene flow. The maintenance of the species despite frequent hybridization at present-day hybrid zones is discussed. -

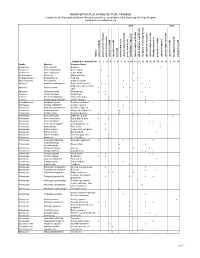
Pinery Provincial Park Vascular Plant List Flowering Latin Name Common Name Community Date
Pinery Provincial Park Vascular Plant List Flowering Latin Name Common Name Community Date EQUISETACEAE HORSETAIL FAMILY Equisetum arvense L. Field Horsetail FF Equisetum fluviatile L. Water Horsetail LRB Equisetum hyemale L. ssp. affine (Engelm.) Stone Common Scouring-rush BS Equisetum laevigatum A. Braun Smooth Scouring-rush WM Equisetum variegatum Scheich. ex Fried. ssp. Small Horsetail LRB Variegatum DENNSTAEDIACEAE BRACKEN FAMILY Pteridium aquilinum (L.) Kuhn Bracken-Fern COF DRYOPTERIDACEAE TRUE FERN FAMILILY Athyrium filix-femina (L.) Roth ssp. angustum (Willd.) Northeastern Lady Fern FF Clausen Cystopteris bulbifera (L.) Bernh. Bulblet Fern FF Dryopteris carthusiana (Villars) H.P. Fuchs Spinulose Woodfern FF Matteuccia struthiopteris (L.) Tod. Ostrich Fern FF Onoclea sensibilis L. Sensitive Fern FF Polystichum acrostichoides (Michaux) Schott Christmas Fern FF ADDER’S-TONGUE- OPHIOGLOSSACEAE FERN FAMILY Botrychium virginianum (L.) Sw. Rattlesnake Fern FF FLOWERING FERN OSMUNDACEAE FAMILY Osmunda regalis L. Royal Fern WM POLYPODIACEAE POLYPODY FAMILY Polypodium virginianum L. Rock Polypody FF MAIDENHAIR FERN PTERIDACEAE FAMILY Adiantum pedatum L. ssp. pedatum Northern Maidenhair Fern FF THELYPTERIDACEAE MARSH FERN FAMILY Thelypteris palustris (Salisb.) Schott Marsh Fern WM LYCOPODIACEAE CLUB MOSS FAMILY Lycopodium lucidulum Michaux Shining Clubmoss OF Lycopodium tristachyum Pursh Ground-cedar COF SELAGINELLACEAE SPIKEMOSS FAMILY Selaginella apoda (L.) Fern. Spikemoss LRB CUPRESSACEAE CYPRESS FAMILY Juniperus communis L. Common Juniper Jun-E DS Juniperus virginiana L. Red Cedar Jun-E SD Thuja occidentalis L. White Cedar LRB PINACEAE PINE FAMILY Larix laricina (Duroi) K. Koch Tamarack Jun LRB Pinus banksiana Lambert Jack Pine COF Pinus resinosa Sol. ex Aiton Red Pine Jun-M CF Pinery Provincial Park Vascular Plant List 1 Pinery Provincial Park Vascular Plant List Flowering Latin Name Common Name Community Date Pinus strobus L. -

An Ethnobotany of Mount Rushmore National Memorial
University of Massachusetts Amherst ScholarWorks@UMass Amherst Landscape Architecture & Regional Planning Masters Projects Landscape Architecture & Regional Planning Summer 2019 An Ethnobotany of Mount Rushmore National Memorial Meredith Savage University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/larp_ms_projects Part of the Landscape Architecture Commons Savage, Meredith, "An Ethnobotany of Mount Rushmore National Memorial" (2019). Landscape Architecture & Regional Planning Masters Projects. 92. Retrieved from https://scholarworks.umass.edu/larp_ms_projects/92 This Article is brought to you for free and open access by the Landscape Architecture & Regional Planning at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Landscape Architecture & Regional Planning Masters Projects by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. AN ETHNOBOTANY OF MOUNT RUSHMORE NATIONAL MEMORIAL Department of Landscape Architecture and Regional Planning University of Massachusetts Amherst Master of Regional Planning Project Meredith S. Savage July 2019 © Copyright by Meredith S. Savage 2019 All Rights Reserved AN ETHNOBOTANY OF MOUNT RUSHMORE NATIONAL MEMORIAL Department of Landscape Architecture and Regional Planning University of Massachusetts Amherst Master of Regional Planning Project Meredith S. Savage July 2019 Approved as to style and content by: __________________________________________ Dr. Elizabeth Brabec, -

The Asparagus Genome Sheds Light on the Origin and Evolution of a Young Y Chromosome
ARTICLE DOI: 10.1038/s41467-017-01064-8 OPEN The asparagus genome sheds light on the origin and evolution of a young Y chromosome Alex Harkess et al.# Sex chromosomes evolved from autosomes many times across the eukaryote phylogeny. Several models have been proposed to explain this transition, some involving male and female sterility mutations linked in a region of suppressed recombination between X and 1234567890 Y (or Z/W, U/V) chromosomes. Comparative and experimental analysis of a reference genome assembly for a double haploid YY male garden asparagus (Asparagus officinalis L.) individual implicates separate but linked genes as responsible for sex determination. Dioecy has evolved recently within Asparagus and sex chromosomes are cytogenetically identical with the Y, harboring a megabase segment that is missing from the X. We show that deletion of this entire region results in a male-to-female conversion, whereas loss of a single sup- pressor of female development drives male-to-hermaphrodite conversion. A single copy anther-specific gene with a male sterile Arabidopsis knockout phenotype is also in the Y-specific region, supporting a two-gene model for sex chromosome evolution. Correspondence and requests for materials should be addressed to Y.Y. (email: [email protected]) or to J.H.L.-M. (email: [email protected]) or to G.C. (email: [email protected]). #A full list of authors and their affliations appears at the end of the paper NATURE COMMUNICATIONS | 8: 1279 | DOI: 10.1038/s41467-017-01064-8 | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | DOI: 10.1038/s41467-017-01064-8 ver the last century, cytological and genetic evidence has development and promote male function. -

Herbivory Affects Male and Female Reproductive Success Differently In
DOI: 10.1111/eea.12337 15TH INTERNATIONAL SYMPOSIUM ON INSECT-PLANT RELATIONSHIPS Herbivory affects male and female reproductive success differently in dioecious Silene latifolia Maria Litto1, Giovanni Scopece1,2*, Silvia Fineschi2, Florian P.Schiestl3 & Salvatore Cozzolino1,2 1Department of Biology, University of Naples Federico II, Complesso Universitario MSA, Via Cinthia, I-80126, Naples, Italy, 2Institute for Sustainable Plant Protection, Consiglio Nazionale delle Ricerche, Via Madonna del Piano 10, I-50019, Sesto Fiorentino, Italy, and 3Institute of Systematic Botany, University of Zurich,€ Zollikerstrasse 107, CH-8008, Zurich€ Switzerland Accepted: 30 March 2015 Key words: floral traits, flower number, phenology, resource allocation, reproductive efforts, Spodoptera littoralis, Lepidoptera, Noctuidae, Caryophyllaceae Abstract In dioecious plants, reproductive efforts of male and female plants can be differentially affected by herbivory due to sex-specific allocation and re-modulation of resources. Here, we investigate the effects of foliar herbivory by the polyphagous species Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) on male and female reproductive traits of Silene latifolia (Poir.) (Caryophyllaceae). We set up experimental populations of male and female control and Spodoptera-infested plants and esti- mated flower morphology and phenology, and plant reproductive success. Herbivory did not affect floral traits involved in visual signalling to pollinators (flower size). Female reproductive success did not differ between Spodoptera-infested and control plants in terms of seed set and seed germination. On the other hand, Spodoptera-infested male plants produced fewer flowers than male control plants and maintained fewer flowers during flowering peaks. By experimental reduction in male flower number, we showed that male flower number is directly correlated with male reproductive success (pollen export to female flowers). -

Enemy Release Mitigates Inbreeding Depression in Native and Invasive Silene Latifolia
bioRxiv preprint doi: https://doi.org/10.1101/401430; this version posted August 28, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 1 Title 2 Enemy release mitigates inbreeding depression in native and invasive Silene latifolia 3 populations: experimental insight into the role of inbreeding × environment interactions in 4 invasion success 5 Running title: 6 I×E interactions in a plant invader 7 Type of article 8 Research article 9 Authors 10 Karin Schrieber1,2, Sabrina Wolf2, Catherina Wypior2, Diana Höhlig2, Stephen R. Keller3, 11 Isabell Hensen2,4, Susanne Lachmuth2,4 12 1Bielefeld University, Faculty of Biology, Department of Chemical Ecology, 33615 Bielefeld, 13 Germany 14 2Martin-Luther-University Halle-Wittenberg, Institute of Biology, Geobotany & Botanical 15 Garden, 06108 Halle (Saale), Germany 16 3University of Vermont, Department of Plant Biology, Burlington, VT 05405, U.S.A. 17 4German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, 04103 18 Leipzig, Germany 19 Corresponding author 20 Karin Schrieber 21 Bielefeld University, Faculty of Biology, Department of Chemical Ecology 22 Universitätsstraße 25 23 D-33615 Bielefeld bioRxiv preprint doi: https://doi.org/10.1101/401430; this version posted August 28, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 2 24 GERMANY 25 Phone: +49 (0) 521 106 67317 26 E-mail: [email protected] 27 No. of 28 Words (total): 8736 29 Words (main body): 5138 30 Figures: 4 31 Tables: 1 32 References: 76 33 Supporting information: 2 34 Provision of data 35 All datasets that support this article will be deposited in Dryad in case the article should be 36 editorially accepted for publication. -

From Cacti to Carnivores: Improved Phylotranscriptomic Sampling And
Article Type: Special Issue Article RESEARCH ARTICLE INVITED SPECIAL ARTICLE For the Special Issue: Using and Navigating the Plant Tree of Life Short Title: Walker et al.—Phylotranscriptomic analysis of Caryophyllales From cacti to carnivores: Improved phylotranscriptomic sampling and hierarchical homology inference provide further insight into the evolution of Caryophyllales Joseph F. Walker1,13, Ya Yang2, Tao Feng3, Alfonso Timoneda3, Jessica Mikenas4,5, Vera Hutchison4, Caroline Edwards4, Ning Wang1, Sonia Ahluwalia1, Julia Olivieri4,6, Nathanael Walker-Hale7, Lucas C. Majure8, Raúl Puente8, Gudrun Kadereit9,10, Maximilian Lauterbach9,10, Urs Eggli11, Hilda Flores-Olvera12, Helga Ochoterena12, Samuel F. Brockington3, Michael J. Moore,4 and Stephen A. Smith1,13 Manuscript received 13 October 2017; revision accepted 4 January 2018. 1 Department of Ecology & Evolutionary Biology, University of Michigan, 830 North University Avenue, Ann Arbor, MI 48109-1048 USA 2 Department of Plant and Microbial Biology, University of Minnesota-Twin Cities, 1445 Gortner Avenue, St. Paul, MN 55108 USA 3 Department of Plant Sciences, University of Cambridge, Cambridge CB2 3EA, UK 4 Department of Biology, Oberlin College, Science Center K111, 119 Woodland Street, Oberlin, OH 44074-1097 USA 5 Current address: USGS Canyonlands Research Station, Southwest Biological Science Center, 2290 S West Resource Blvd, Moab, UT 84532 USA 6 Institute of Computational and Mathematical Engineering (ICME), Stanford University, 475 Author Manuscript Via Ortega, Suite B060, Stanford, CA, 94305-4042 USA This is the author manuscript accepted for publication and has undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. -
Preliminary Plant List
Growing Plant List of RoeJan Park, Hillsdale compiled by the Participatory Natural History Survey Group, an initiative of the Farmscape Ecology Program [email protected] 2009 2008 e e d i d i s - s - e n e e e W d a d d d W w i w i i i J e s o o s s s d - e g - - - d d w a o d W S a a S S E W a o o e f R t e e r t l E d d s w w t w w s w f E s E E a a a M m W d s o f e o o o o o e w m l N l e o N N r r r r r t r r r e r d r e o a t r e t t e e e e s o e e i a o a m r f d s f f s s o e g g g F g e / w S t w m f y o a e r e e d d r d d a e r d o o r r a e n h o m l l n l e n e a e i e e i o f f i o o h s w f e F r d f a h m h h f f h y i d a l d r f a d l l t l n n n n b p i p i a p d d d d d d d r r r r a n p a c b t y d n n n n n n i n e e e e f m w a i m W d l t t u l t t n a a a a a o a a a n t a b r l o l l l l l l r a s s s s o c f e W a h w W p o p p p a p l p i p a a a a w l l N u f e e H S w u u u e e b u s a S F u S p u Habitat # on Aerial Photo 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 20 15 5 18 19 Family Species Common Name Aceraceae Acer negundo Boxelder x Aceraceae Acer saccharinum Silver maple x Aceraceae Acer saccharum Sugar maple x Alismataceae Alisma sp. -

WRA Species Report
Family: Caryophyllaceae Taxon: Silene armeria Synonym: NA Common Name: sweet William catchfly Sweet William campion Questionaire : current 20090513 Assessor: Chuck Chimera Designation: H(HPWRA) Status: Assessor Approved Data Entry Person: Chuck Chimera WRA Score 7 101 Is the species highly domesticated? y=-3, n=0 n 102 Has the species become naturalized where grown? y=1, n=-1 103 Does the species have weedy races? y=1, n=-1 201 Species suited to tropical or subtropical climate(s) - If island is primarily wet habitat, then (0-low; 1-intermediate; 2- Low substitute "wet tropical" for "tropical or subtropical" high) (See Appendix 2) 202 Quality of climate match data (0-low; 1-intermediate; 2- Low high) (See Appendix 2) 203 Broad climate suitability (environmental versatility) y=1, n=0 y 204 Native or naturalized in regions with tropical or subtropical climates y=1, n=0 205 Does the species have a history of repeated introductions outside its natural range? y=-2, ?=-1, n=0 y 301 Naturalized beyond native range y = 1*multiplier (see y Appendix 2), n= question 205 302 Garden/amenity/disturbance weed n=0, y = 1*multiplier (see y Appendix 2) 303 Agricultural/forestry/horticultural weed n=0, y = 2*multiplier (see Appendix 2) 304 Environmental weed n=0, y = 2*multiplier (see n Appendix 2) 305 Congeneric weed n=0, y = 1*multiplier (see y Appendix 2) 401 Produces spines, thorns or burrs y=1, n=0 n 402 Allelopathic y=1, n=0 n 403 Parasitic y=1, n=0 n 404 Unpalatable to grazing animals y=1, n=-1 405 Toxic to animals y=1, n=0 406 Host for recognized -

Phylogenetic Relationships of Silene Sect. Melandrium and Allied Taxa
! "#! $ %&'(%)($ *++%,-,./,0 *+)%12.,.--0.1-,,. ! 3 4 3 3!!35.,6626 !" # $%&%&&'&'()&* * * + ,- . , / ",%&&',+ / *, " - 0 1 * $ 2 -," , 3)',4& , ,'5!6' 6774657 63, -* *8 0 1 " 9 * : * , * 8 ," . ; * : * , * * 0 1,- * * ,- " ,- ; : * . , < . ** * =>? . 6 +/ ,- ; , " * , ! , $ 8* " ,@ * * ,* ! * , - . * **, "# $= $ = =-"=- " "/" /+"%/+%/+%=> =? <-= @ %&' ( ) *+,-./# A" 8/ %&&' <== 37 63% 4 <='5!6' 6774657 63 ( ((( 6 &&!&30 (BB ,:,B C D ( ((( 6 &&!&31 Cover illustrations: Barry Breckling, Sara Gold, Kjell Lännerholm, Anja Rautenberg and Wiebke Rautenberg. List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I Rautenberg, A., Filatov, D., Svennblad, B., Heidari, N., Oxel- man, B. (2008) Conflicting phylogenetic signals in the SlX1/Y1 gene in Silene. BMC Evolutionary Biology, 8:299 II Rautenberg, A., Hathaway, L., Oxelman, B., Prentice, H. C. Phylogenetic relationships of Silene section Elisanthe (Caryo- phyllaceae) as inferred from chloroplast and nuclear DNA se- quences. Manuscript. III Rautenberg, A., Hathaway, L., Prentice, H. C., Oxelman, B. Phylogenetic relationships