1563.Full-Text.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Utility of the Digital Rectal Examination in the Emergency Department: a Review
The Journal of Emergency Medicine, Vol. 43, No. 6, pp. 1196–1204, 2012 Published by Elsevier Inc. Printed in the USA 0736-4679/$ - see front matter http://dx.doi.org/10.1016/j.jemermed.2012.06.015 Clinical Reviews UTILITY OF THE DIGITAL RECTAL EXAMINATION IN THE EMERGENCY DEPARTMENT: A REVIEW Chad Kessler, MD, MHPE*† and Stephen J. Bauer, MD† *Department of Emergency Medicine, Jesse Brown VA Medical Center and †University of Illinois-Chicago College of Medicine, Chicago, Illinois Reprint Address: Chad Kessler, MD, MHPE, Department of Emergency Medicine, Jesse Brown Veterans Hospital, 820 S Damen Ave., M/C 111, Chicago, IL 60612 , Abstract—Background: The digital rectal examination abdominal pain and acute appendicitis. Stool obtained by (DRE) has been reflexively performed to evaluate common DRE doesn’t seem to increase the false-positive rate of chief complaints in the Emergency Department without FOBTs, and the DRE correlated moderately well with anal knowing its true utility in diagnosis. Objective: Medical lit- manometric measurements in determining anal sphincter erature databases were searched for the most relevant arti- tone. Published by Elsevier Inc. cles pertaining to: the utility of the DRE in evaluating abdominal pain and acute appendicitis, the false-positive , Keywords—digital rectal; utility; review; Emergency rate of fecal occult blood tests (FOBT) from stool obtained Department; evidence-based medicine by DRE or spontaneous passage, and the correlation be- tween DRE and anal manometry in determining anal tone. Discussion: Sixteen articles met our inclusion criteria; there INTRODUCTION were two for abdominal pain, five for appendicitis, six for anal tone, and three for fecal occult blood. -

HIGH-SENSITIVITY GUAIAC-BASED FECAL OCCULT BLOOD TEST (HSGFOBT) Anyone Can Get Colon Cancer
GET SCREENED: DETECT ANDDETECT PREVENT AND PREVENT COLON CANCER COLON CANCER TEST TYPE: HIGH-SENSITIVITY GUAIAC-BASED FECAL OCCULT BLOOD TEST (HSGFOBT) Anyone can get colon cancer. It can affect people of all racial and ethnic groups. Routine screening can help your health care provider find cancers earlier, when they are easier to treat. Screening may also prevent cancer, by finding and removing polyps or abnormal growths from the colon. COLON CANCER SCREENING There are different test options for screening. Talk with your provider to choose FACT SHEET the test that’s right for you. WHO? Adults who are at average risk for colon WHERE? You do this test at home. cancer may have an HSgFOBT. Talk with your health WHY? HSgFOBT detects signs of colon and rectal care provider about your risk and what age to begin cancer. It can also detect some polyps, which are screening. If you are at an increased risk of colon growths that could become cancer later. cancer, you may need screening early or this test may not be right for you. Discuss your medical and family HOW? You may have a restricted diet starting a medical history with your provider before choosing a few days before the test. You will get a kit from your test. Tell them if you have any of these risk factors: provider with instructions about how to take a sample A history of colon cancer or precancerous polyps of stool from three bowel movements in a row that A parent, sibling or child with colon cancer or you collect into a clean container. -

Unenhanced Areas Revealed by Contrast-Enhanced Abdominal Ultrasonography with Sonazoidtm Potentially Correspond to Colorectal Cancer
4012 EXPERIMENTAL AND THERAPEUTIC MEDICINE 12: 4012-4016, 2016 Unenhanced areas revealed by contrast-enhanced abdominal ultrasonography with SonazoidTM potentially correspond to colorectal cancer MINORU TOMIZAWA1, MIZUKI TOGASHI2, FUMINOBU SHINOZAKI3, RUMIKO HASEGAWA4, YOSHINORI SHIRAI4, MIDORI NORITAKE2, YUKIE MATSUOKA2, HIROAKI KAINUMA2, YASUJI IWASAKI2, KAZUNORI FUGO5, YASUFUMI MOTOYOSHI6, TAKAO SUGIYAMA7, SHIGENORI YAMAMOTO8, TAKASHI KISHIMOTO5 and NAOKI ISHIGE9 Departments of 1Gastroenterology; 2Clinical Laboratory; 3Radiology and 4Surgery, National Hospital Organization, Shimoshizu Hospital, Yotsukaido City, Chiba 284-0003; 5Department of Molecular Pathology, Chiba University Graduate School of Medicine, Chiba City, Chiba 260-8670; Departments of 6Neurology; 7Rheumatology; 8Pediatrics and 9Neurosurgery, National Hospital Organization, Shimoshizu Hospital, Yotsukaido, Yotsukaido City, Chiba 284-0003, Japan Received September 18, 2015; Accepted September 22, 2016 DOI: 10.3892/etm.2016.3868 Abstract. The present study investigated the potential utility of Introduction contrast-enhanced abdominal ultrasonography (CEUS), using SonazoidTM, in colorectal cancer (CRC). Three patients were Colorectal cancer (CRC) is commonly observed in clinical subjected to CEUS with SonazoidTM. Surgical specimens were settings (1). To improve the prognosis in patients with CRC, immunostained for CD31. Numbers of blood vessels positive prompt and accurate diagnosis is essential. Screening for CRC for CD31 were analyzed in each of five fields at x400 magnifi- is performed using fecal occult blood testing, and is diagnosed cation and averaged to determine blood vessel density. Blood with colonoscopy (2). vessel density was compared between non-tumorous and Abdominal ultrasound (US) is useful for the safe and tumorous areas. Prior to the administration of SonazoidTM, easy diagnosis of patients (3-6). During US screening of the CRC was illustrated as irregular-shaped wall thickening. -

Flexible Sigmoidoscopy in Asymptomatic Patients with Negative Fecal Occult Blood Tests Joy Garrison Cauffman, Phd, Jimmy H
Flexible Sigmoidoscopy in Asymptomatic Patients with Negative Fecal Occult Blood Tests Joy Garrison Cauffman, PhD, Jimmy H. Hara, MD, Irving M. Rasgon, MD, and Virginia A. Clark, PhD Los Angeles, California Background. Although the American Cancer Society and tients with lesions were referred for colonoscopy; addi others haw established guidelines for colorectal cancer tional lesions were found in 14%. A total of 62 lesions screening, questions of who and how to screen still exist. were discovered, including tubular adenomas, villous Methods. A 60-crn flexible sigmoidoscopy was per adenomas, tubular villous adenomas (23 of the adeno formed on 1000 asymptomatic patients, 45 years of mas with atypia), and one adenocarcinoma. The high age or older, with negative fecal occult blood tests, est percentage of lesions discovered were in the sig who presented for routine physical examinations. Pa moid colon and the second highest percentage were in tients with clinically significant lesions were referred for the ascending colon. colonoscopy. The proportion of lesions that would not Conclusions. The 60-cm flexible sigmoidoscope was have been found if the 24-cm rigid or the 30-cm flexi able to detect more lesions than either the 24-cm or ble sigmoidoscope had been used was identified. 30-cm sigmoidoscope when used in asymptomatic pa Results. Using the 60-cm flexible sigmoidoscope, le tients, 45 years of age and over, with negative fecal oc sions were found in 3.6% of the patients. Eighty per cult blood tests. When significant lesions are discovered cent of the significant lesions were beyond the reach of by sigmoidoscopy, colonoscopy should be performed. -

ASMBS Position Statement on the Relationship Between Obesity And
Surgery for Obesity and Related Diseases 16 (2020) 713–724 ASMBS Guidelines/Statements ASMBS position statement on the relationship between obesity and cancer, and the role of bariatric surgery: risk, timing of treatment, effects on disease biology, and qualification for surgery Saber Ghiassi, M.D.a, Maher El Chaar, M.D.b, Essa M. Aleassa, M.D.c,d, Fady Moustarah, M.D.e, Sofiane El Djouzi, M.D.f, T. Javier Birriel, M.D.g, Ann M. Rogers, M.D.h,*, for the American Society for Metabolic and Bariatric Surgery Clinical Issues Committee aDepartment of Surgery, Yale University School of Medicine, New Haven, Connecticut bDepartment of Bariatric Surgery, St. Luke’s University Health Network, Allentown, Pennsylvania cDepartment of General Surgery, Bariatric and Metabolic Institute, Cleveland Clinic, Cleveland, Ohio dDepartment of Surgery, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates eAscension St. Mary’s Hospital, Saginaw, Michigan fAMITA Health, Hoffman Estates, Illinois gSt. Luke’s University Health Network, Stroudsburg, Pennsylvania hDivision of Minimally Invasive and Bariatric Surgery, Penn State Health, Hershey, Pennsylvania Received 13 March 2020; accepted 16 March 2020 Preamble bariatric surgery. In this statement, a summary of current, published, peer-reviewed scientific evidence, and expert The following position statement is issued by the Amer- opinion is presented. The intent of issuing such a statement ican Society for Metabolic and Bariatric Surgery in response is to provide available objective information about these to numerous inquiries made to the Society by patients, phy- topics. The statement is not intended as, and should not be sicians, Society members, hospitals, health insurance construed as, stating or establishing a local, regional, or na- payors, the media, and others, regarding the relationship be- tional standard of care. -

High Quality Fecal Occult Blood Testing (FOBT) for CRC Screening: Evidence and Recommendations
High Quality Fecal Occult Blood Testing (FOBT) for CRC Screening: Evidence and Recommendations Rationale for use of FOBT High sensitivity fecal occult blood testing (FOBT) is one of the colorectal cancer screening methods recommended in guidelines from the American Cancer Society, the US Preventive Services Taskforce, and every other major medical organization. In spite of this widespread endorsement, primary care clinicians often express conviction that colonoscopy is the “gold standard” test for colorectal cancer screening and that the use of FOBT represents sub-standard care. These beliefs persist in spite of well-documented shortcomings of endoscopy (missed adenomas and cancers, higher complication rates and higher one-time costs than other screening methodologies), and the fact that access to endoscopy is limited or non- existent for a significant proportion of the U.S. population. Many clinicians are also unaware that randomized controlled trials of FOBT screening have demonstrated decreases in colorectal cancer incidence and mortality, and modeling studies suggest that the years of life saved through a high quality FOBT screening program are essentially the same as with a high quality colonoscopy based screening programs. Recent advances in stool blood screening include the emergence of new tests and improved understanding of the impact of quality factors on testing outcomes. This document provides state-of-the-science information about high quality stool testing. Types of Fecal Occult Blood Tests Two main types of FOBT are available – guaiac and immunochemical. Both types of FOBT have been shown to have reasonably high detection rates for colon and rectal cancers; adenoma detection rates are appreciably lower. -
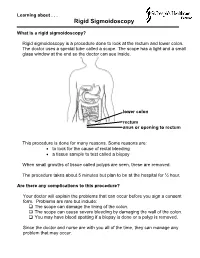
What Is a Rigid Sigmoidoscopy?
Learning about . Rigid Sigmoidoscopy What is a rigid sigmoidoscopy? Rigid sigmoidoscopy is a procedure done to look at the rectum and lower colon. The doctor uses a special tube called a scope. The scope has a light and a small glass window at the end so the doctor can see inside. lower colon rectum anus or opening to rectum This procedure is done for many reasons. Some reasons are: • to look for the cause of rectal bleeding • a tissue sample to test called a biopsy When small growths of tissue called polyps are seen, these are removed. The procedure takes about 5 minutes but plan to be at the hospital for ½ hour. Are there any complications to this procedure? Your doctor will explain the problems that can occur before you sign a consent form. Problems are rare but include: The scope can damage the lining of the colon. The scope can cause severe bleeding by damaging the wall of the colon. You may have blood spotting if a biopsy is done or a polyp is removed. Since the doctor and nurse are with you all of the time, they can manage any problem that may occur. What do I need to do to get ready at home? 4 to 5 days before your procedure: Taking medications: Your doctor may want you to stop taking certain medications 4 to 5 days before the procedure. If you need to stop any medications, your doctor will tell you during the office visit. If you have any questions, call the doctor’s office. Buying a Fleet enema: Your bowel must be clean and empty of waste material before this procedure. -

04. EDITORIAL 1/2/06 10:34 Página 853
04. EDITORIAL 1/2/06 10:34 Página 853 1130-0108/2005/97/12/853-859 REVISTA ESPAÑOLA DE ENFERMEDADES DIGESTIVAS REV ESP ENFERM DIG (Madrid) Copyright © 2005 ARÁN EDICIONES, S. L. Vol. 97, N.° 12, pp. 853-859, 2005 Cost-effectiveness of abdominal ultrasonography in the diagnosis of colorectal carcinoma Colorectal cancer (CRC) is a most common neoplasm, and the second leading cause of cancer-related death. CRC was responsible for 11% of cancer-related deaths in males, and for 15% of cancer-related deaths in females according to data for year 2000. Most recent data reported in Spain on death causes in 2002 suggest that CRC was responsible for 12,183 deaths (6,896 males with a mean age of 70 years, and 5,287 women with a mean age of 71 years). In these tumors, mortality data do not reflect the true incidence of this disease, since survival has improved in recent years, particularly in younger individuals. In contrast to other European countries, Spain ranks in an intermediate position in terms of CRC-re- lated incidence and mortality. This risk clearly increases with age, with a notori- ous rise in incidence from 50 years of age on. Survival following CRC detection and management greatly depends upon tumor stage at the time of diagnosis; hence the importance of early detection and –because of their malignant poten- tial– of the recognition and excision of colorectal adenomas. Thus, polypectomy and then surveillance are the primary cornerstones in the prevention of CRC (1-4). For primary prevention, fiber-rich diets, physical exercising, and the avoidance of overweight, smoking, and alcohol have been recommended. -

Flexible Sigmoidoscopy with Haemorrhoid Banding
Flexible sigmoidoscopy with haemorrhoid banding You have been referred by your doctor to have a flexible sigmoidoscopy which may also include haemorrhoid banding. If you are unable to keep your appointment, please notify the department as soon as possible. This will allow staff to give your appointment to someone else and they will arrange another date and time for you. This booklet has been written to explain the procedures. This will help you make an informed decision in relation to consenting to the investigation. Please read the booklets and consent form carefully. You will need to complete the enclosed questionnaire. You may be contacted via telephone by a trained endoscopy nurse before the procedure, to go through the admission process and answer any queries you may have. If you are not contacted please come to your appointment at the time stated on your letter. If you have any mobility problems or there is a possibility you could be pregnant please telephone appointments staff on 01284 712748. Please note the appointment time is your arrival time on the unit, and not the time of your procedure. Please remember there will be other patients in the unit who arrive after you, but are taken in for their procedure before you. This is either for medical reasons or they are seeing a different Doctor. Due to the limited space available and to maintain other patients’ privacy and dignity, we only allow patients (and carers) through to the ward area. Relatives/escorts will be contacted once the person is available for collection. The Endoscopy Unit endeavours to offer single sex facilities, and we aim to make your stay as comfortable and stress free as possible. -

Flexible Sigmoidoscopy – Outpatients
Flexible sigmoidoscopy – Outpatients You have been referred by your doctor to have a flexible sigmoidoscopy. his information booklet has been written to explain the procedure. This will help you to make an informed decision before consenting to the procedure. If you are unable to attend your appointment please inform us as soon as possible. Please ensure you read this booklet and the enclosed consent form thoroughly. Please also complete the enclosed health questionnaire. You may be contacted by an endoscopy trained nurse before your procedure to go through the admission process and answer any queries you may have. If you are not contacted please come to your appointment at the time stated in your letter. Please note your appointment time is your arrival time on the unit and not the time of your procedure. If you have any mobility issues or if there is a possibility you could be pregnant, please contact the appointment staff on 01284 713551 Please remember there will be other patients in the unit who may arrive after you but are taken in for their procedure before you, this is for medical reasons or they are seeing a different doctor. Due to limited space available and to maintain other patients’ privacy and dignity, we only allow patients (and carers) through to the ward area. Relatives/ escorts will be contacted once you are ready for collection. The Endoscopy Unit endeavours to offer single sex facilities, and we aim to make you stay as comfortable and stress free as possible. Source: Endoscopy Reference No: 5035-14 Issue date: 8/1/21 Review date: 8/1/24 Page 1 of 11 Medication If you are taking WARFARIN, CLOPIDOGREL, RIVAROXABAN or any other anticoagulant (blood thinning medication), please contact the appointment staff on 01284 713551, your GP or anticoagulation nurse, as special arrangements may be necessary. -

Anesthesiology Services for Gastrointestinal Endoscopy Reference Number: CP.MP.161 Coding Implications Last Review Date: 05/19 Revision Log
Clinical Policy: Anesthesiology Services for Gastrointestinal Endoscopy Reference Number: CP.MP.161 Coding Implications Last Review Date: 05/19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Conscious sedation for gastrointestinal (GI) endoscopic procedures is standard of care to relieve patient anxiety and discomfort, improve outcomes of the examination, and decrease the memory of the procedure. A combination of an opioid and benzodiazepine is the recommended regimen for achieving minimal to moderate sedation for upper endoscopy and colonoscopy in people without risk for sedation-related adverse events.5 Generally, the gastroenterologist performing the procedure and/or his/her qualified assistant can adequately manage the administration of conscious sedation and monitoring of the patient. In cases with sedation-related risk factors, additional assistance from an anesthesia team member is required to ensure the safest outcome for the patient. This policy outlines the indications for which anesthesia services are considered medically necessary. Policy/Criteria I. It is the policy of health plans affiliated with Centene Corporation® that anesthesiology services for GI endoscopic procedures is considered medically necessary for the following indications: A. Age < 18 years or ≥ 70 years; B. Pregnancy; C. Increased risk of complications due to physiological status as identified by the American Society of Anesthesiologist (ASA) physical status classification of ASA III or higher; D. Increased risk for airway obstruction because of anatomic variants such as dysmorphic facial features, oral abnormalities, neck abnormalities, or jaw abnormalities; E. History of or anticipated intolerance to conscious sedation (i.e. chronic opioid or benzodiazepine use); F. -

Fecal Occult Blood Test CPT: 82272
Medicare National Coverage Determination Policy Fecal Occult Blood Test CPT: 82272 CMS National Coverage Policy Coverage Indications, Limitations, and/or Medical Necessity The Fecal Occult Blood Test (FOBT) detects the presence of trace amounts of blood in stool. The procedure is performed by testing one or several small samples of one, two or three different stool specimens. This test may be performed with or without evidence of iron deficiency anemia, which may be related to gastrointestinal blood loss. The range of causes for blood loss include inflammatory causes, including acid-peptic disease, non-steroidal anti-inflammatory drug use, hiatal hernia, Crohn’s disease, ulcerative colitis, gastroenteritis, and colon ulcers. It is also seen with infectious causes, including hookworm, strongyloides, ascariasis, tuberculosis, and enteroamebiasis. Vascular causes include angiodysplasia, hemangiomas, varices, blue rubber bleb nevus syndrome, and watermelon stomach. Tumors and neoplastic causes include lymphoma, leiomyosarcoma, lipomas, adenocarcinoma and primary and secondary metastases to the GI tract. Drugs such as nonsteroidal anti-inflammatory drugs also cause bleeding. There are extra gastrointestinal causes such as hemoptysis, epistaxis, and oropharyngeal bleeding. Artifactual causes include hematuria, and menstrual bleeding. In addition, there may be other causes such as coagulopathies, gastrostomy tubes or other appliances, factitial causes, and long distance running. Three basic types of fecal hemoglobin assays exist, each directed at a different component of the hemoglobin molecule. 1. Immunoassays recognize antigenic sites on the globin portion and are least affected by diet or proximal gut bleeding, but the antigen may be destroyed by fecal flora. 2. The heme-porphyrin assay measures heme-derived porphyrin and is least influenced by enterocolic metabolism or fecal storage.