Are Proximal Colorectal Cancers Always Associated with Distal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review Article Laparoscopic Versus Open Live Donor Hepatectomy in Liver Transplantation: a Systemic Review and Meta-Analysis
Int J Clin Exp Med 2016;9(8):15004-15016 www.ijcem.com /ISSN:1940-5901/IJCEM0021495 Review Article Laparoscopic versus open live donor hepatectomy in liver transplantation: a systemic review and meta-analysis Dong-Wei Xu*, Ping Wan*, Jian-Jun Zhang, Qiang Xia Department of Liver Surgery, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China. *Equal contributors. Received December 9, 2015; Accepted March 19, 2016; Epub August 15, 2016; Published August 30, 2016 Abstract: Objective: The aim of this study was to compare laparoscopic versus open live donor liver transplantation using meta-analysis. Background: Living donor liver transplantation (LDLT), as an alternative to deceased donor liver transplantation (DDLT), has increasingly performed all around the world. Laparoscopic live donor hepatectomy (LLDH) has been performed increasingly, and is gaining worldwide acceptance. As the studies assessing the safety and efficacy of laparoscopic compared with open techniques is growing, we combined the available data to conduct this meta-analysis to compare the two techniques. Methods: A literature search was performed to identify studies comparing laparoscopic with open live donor hepatectomy (OLDH) published before June 2015. Perioperative out- comes (blood loss, operative time, hospital stay, analgesia use) and postoperative complications (donors and reci- pients postoperative complications, recipients specific postoperative complications including biliary complications and vascular complications) were the main outcomes evaluated in the meta-analysis. Results: Fourteen studies with a total of 1136 patients were included in this meta-analysis, of which 357 were treated by laparoscopic technique and 779 were treated by the open procedures. Compared with the open group, laparoscopic group was associated with significant less estimated blood loss (P=0.01), shorter duration of operation (P=0.02), length of hospital stay (P=0.003) and duration of PCA use (P=0.04). -

Flexible Sigmoidoscopy in Asymptomatic Patients with Negative Fecal Occult Blood Tests Joy Garrison Cauffman, Phd, Jimmy H
Flexible Sigmoidoscopy in Asymptomatic Patients with Negative Fecal Occult Blood Tests Joy Garrison Cauffman, PhD, Jimmy H. Hara, MD, Irving M. Rasgon, MD, and Virginia A. Clark, PhD Los Angeles, California Background. Although the American Cancer Society and tients with lesions were referred for colonoscopy; addi others haw established guidelines for colorectal cancer tional lesions were found in 14%. A total of 62 lesions screening, questions of who and how to screen still exist. were discovered, including tubular adenomas, villous Methods. A 60-crn flexible sigmoidoscopy was per adenomas, tubular villous adenomas (23 of the adeno formed on 1000 asymptomatic patients, 45 years of mas with atypia), and one adenocarcinoma. The high age or older, with negative fecal occult blood tests, est percentage of lesions discovered were in the sig who presented for routine physical examinations. Pa moid colon and the second highest percentage were in tients with clinically significant lesions were referred for the ascending colon. colonoscopy. The proportion of lesions that would not Conclusions. The 60-cm flexible sigmoidoscope was have been found if the 24-cm rigid or the 30-cm flexi able to detect more lesions than either the 24-cm or ble sigmoidoscope had been used was identified. 30-cm sigmoidoscope when used in asymptomatic pa Results. Using the 60-cm flexible sigmoidoscope, le tients, 45 years of age and over, with negative fecal oc sions were found in 3.6% of the patients. Eighty per cult blood tests. When significant lesions are discovered cent of the significant lesions were beyond the reach of by sigmoidoscopy, colonoscopy should be performed. -

Management of Autoimmune Liver Diseases After Liver Transplantation
Review Management of Autoimmune Liver Diseases after Liver Transplantation Romelia Barba Bernal 1,† , Esli Medina-Morales 1,† , Daniela Goyes 2 , Vilas Patwardhan 1 and Alan Bonder 1,* 1 Division of Gastroenterology and Hepatology, Beth Israel Deaconess Medical Center, Boston, MA 02215, USA; [email protected] (R.B.B.); [email protected] (E.M.-M.); [email protected] (V.P.) 2 Department of Medicine, Loyola Medicine—MacNeal Hospital, Berwyn, IL 60402, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-617-632-1070 † These authors contributed equally to this project. Abstract: Autoimmune liver diseases are characterized by immune-mediated inflammation and even- tual destruction of the hepatocytes and the biliary epithelial cells. They can progress to irreversible liver damage requiring liver transplantation. The post-liver transplant goals of treatment include improving the recipient’s survival, preventing liver graft-failure, and decreasing the recurrence of the disease. The keystone in post-liver transplant management for autoimmune liver diseases relies on identifying which would be the most appropriate immunosuppressive maintenance therapy. The combination of a steroid and a calcineurin inhibitor is the current immunosuppressive regimen of choice for autoimmune hepatitis. A gradual withdrawal of glucocorticoids is also recommended. Citation: Barba Bernal, R.; On the other hand, ursodeoxycholic acid should be initiated soon after liver transplant to prevent Medina-Morales, E.; Goyes, D.; recurrence and improve graft and patient survival in primary biliary cholangitis recipients. Unlike the Patwardhan, V.; Bonder, A. Management of Autoimmune Liver previously mentioned autoimmune diseases, there are not immunosuppressive or disease-modifying Diseases after Liver Transplantation. -

Liver Transplantation As Last-Resort Treatment for Patients with Bile Duct Injuries Following Cholecystectomy: a Multicenter Analysis
ORIGINAL ARTICLE Annals of Gastroenterology (2020) 33, 1-8 Liver transplantation as last-resort treatment for patients with bile duct injuries following cholecystectomy: a multicenter analysis Peter Tsaparasa, Nikolaos Machairasa, Victoria Ardilesb, Marek Krawczykc, Damiano Patronod, Umberto Baccaranie, Umberto Cillof, Einar Martin Aandahlg, Christian Cotsoglouh, Johana Leiva Espinozab, Rodrigo Sanchez Claríab, Ioannis D. Kostakisa, Aksel Fossg, Vincenzo Mazzaferroh, Eduardo de Santibañesb, Georgios C. Sotiropoulosa,i Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece; Hospital Italiano de Buenos Aires, Buenos Aires, Argentina; Medical University of Warsaw, Poland; University of Torino, Turin, Italy; University of Udine, Udine, Italy; University of Padova School of Medicine, Padova, Italy; Oslo University Hospital, Oslo, Norway; University of Milan, Milan, Italy; University Hospital Essen, Germany Abstract Background Liver transplantation (LT) has been used as a last resort in patients with end-stage liver disease due to bile duct injuries (BDI) following cholecystectomy. Our study aimed to identify and evaluate factors that cause or contribute to an extended liver disease that requires LT as ultimate solution, after BDI during cholecystectomy. Methods Data from 8 high-volume LT centers relating to patients who underwent LT after suffering BDI during cholecystectomy were prospectively collected and retrospectively analyzed. Results Thirty-four patients (16 men, 18 women) with a median age of 45 (range 22-69) years were included in this study. Thirty of them (88.2%) underwent LT because of liver failure, most commonly as a result of secondary biliary cirrhosis. The median time interval between BDI and LT was 63 (range 0-336) months. There were 23 cases (67.6%) of postoperative morbidity, 6 cases (17.6%) of post-transplant 30-day mortality, and 10 deaths (29.4%) in total after LT. -
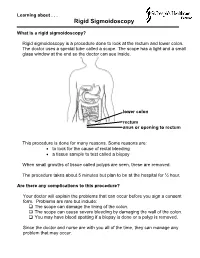
What Is a Rigid Sigmoidoscopy?
Learning about . Rigid Sigmoidoscopy What is a rigid sigmoidoscopy? Rigid sigmoidoscopy is a procedure done to look at the rectum and lower colon. The doctor uses a special tube called a scope. The scope has a light and a small glass window at the end so the doctor can see inside. lower colon rectum anus or opening to rectum This procedure is done for many reasons. Some reasons are: • to look for the cause of rectal bleeding • a tissue sample to test called a biopsy When small growths of tissue called polyps are seen, these are removed. The procedure takes about 5 minutes but plan to be at the hospital for ½ hour. Are there any complications to this procedure? Your doctor will explain the problems that can occur before you sign a consent form. Problems are rare but include: The scope can damage the lining of the colon. The scope can cause severe bleeding by damaging the wall of the colon. You may have blood spotting if a biopsy is done or a polyp is removed. Since the doctor and nurse are with you all of the time, they can manage any problem that may occur. What do I need to do to get ready at home? 4 to 5 days before your procedure: Taking medications: Your doctor may want you to stop taking certain medications 4 to 5 days before the procedure. If you need to stop any medications, your doctor will tell you during the office visit. If you have any questions, call the doctor’s office. Buying a Fleet enema: Your bowel must be clean and empty of waste material before this procedure. -

The Evolution of Minimally Invasive Surgery in Liver Transplantation for Hepatocellular Carcinoma
Sioutas et al. Hepatoma Res 2021;7:26 Hepatoma Research DOI: 10.20517/2394-5079.2020.111 Review Open Access The evolution of minimally invasive surgery in liver transplantation for hepatocellular carcinoma Georgios S. Sioutas1, Georgios Tsoulfas2 1School of Medicine, Democritus University of Thrace, Alexandroupolis 68100, Greece. 2First Department of Surgery, Papageorgiou University Hospital, Aristotle University of Thessaloniki, Thessaloniki 54622, Greece. Correspondence to: Prof. Georgios Tsoulfas, First Department of Surgery, Aristotle University of Thessaloniki, 66 Tsimiski Street, Thessaloniki 54622, Greece. E-mail: [email protected] How to cite this article: Sioutas GS, Tsoulfas G. The evolution of minimally invasive surgery in liver transplantation for hepatocellular carcinoma. Hepatoma Res 2021;7:26. https://dx.doi.org/10.20517/2394-5079.2020.111 Received: 24 Sep 2020 First Decision: 19 Nov 2020 Revised: 22 Nov 2020 Accepted: 26 Nov 2020 Available online: 9 Apr 2021 Academic Editor: Ho-Seong Han Copy Editor: Cai-Hong Wang Production Editor: Jing Yu Abstract Hepatocellular carcinoma (HCC) is a malignant neoplasm associated with significant mortality worldwide. The most commonly applied curative options include liver resection and liver transplantation (LT). Advances in technology have led to the broader implementation of minimally invasive approaches for liver surgery, including laparoscopic, hybrid, hand-assisted, and robotic techniques. Laparoscopic liver resection for HCC or living donor hepatectomy in LT for HCC are considered to be feasible and safe. Furthermore, the combination of laparoscopy and LT is a recent impressive and promising achievement that requires further investigation. This review aims to describe the role of minimally invasive surgery techniques utilized in LT for HCC. -

04. EDITORIAL 1/2/06 10:34 Página 853
04. EDITORIAL 1/2/06 10:34 Página 853 1130-0108/2005/97/12/853-859 REVISTA ESPAÑOLA DE ENFERMEDADES DIGESTIVAS REV ESP ENFERM DIG (Madrid) Copyright © 2005 ARÁN EDICIONES, S. L. Vol. 97, N.° 12, pp. 853-859, 2005 Cost-effectiveness of abdominal ultrasonography in the diagnosis of colorectal carcinoma Colorectal cancer (CRC) is a most common neoplasm, and the second leading cause of cancer-related death. CRC was responsible for 11% of cancer-related deaths in males, and for 15% of cancer-related deaths in females according to data for year 2000. Most recent data reported in Spain on death causes in 2002 suggest that CRC was responsible for 12,183 deaths (6,896 males with a mean age of 70 years, and 5,287 women with a mean age of 71 years). In these tumors, mortality data do not reflect the true incidence of this disease, since survival has improved in recent years, particularly in younger individuals. In contrast to other European countries, Spain ranks in an intermediate position in terms of CRC-re- lated incidence and mortality. This risk clearly increases with age, with a notori- ous rise in incidence from 50 years of age on. Survival following CRC detection and management greatly depends upon tumor stage at the time of diagnosis; hence the importance of early detection and –because of their malignant poten- tial– of the recognition and excision of colorectal adenomas. Thus, polypectomy and then surveillance are the primary cornerstones in the prevention of CRC (1-4). For primary prevention, fiber-rich diets, physical exercising, and the avoidance of overweight, smoking, and alcohol have been recommended. -

Flexible Sigmoidoscopy with Haemorrhoid Banding
Flexible sigmoidoscopy with haemorrhoid banding You have been referred by your doctor to have a flexible sigmoidoscopy which may also include haemorrhoid banding. If you are unable to keep your appointment, please notify the department as soon as possible. This will allow staff to give your appointment to someone else and they will arrange another date and time for you. This booklet has been written to explain the procedures. This will help you make an informed decision in relation to consenting to the investigation. Please read the booklets and consent form carefully. You will need to complete the enclosed questionnaire. You may be contacted via telephone by a trained endoscopy nurse before the procedure, to go through the admission process and answer any queries you may have. If you are not contacted please come to your appointment at the time stated on your letter. If you have any mobility problems or there is a possibility you could be pregnant please telephone appointments staff on 01284 712748. Please note the appointment time is your arrival time on the unit, and not the time of your procedure. Please remember there will be other patients in the unit who arrive after you, but are taken in for their procedure before you. This is either for medical reasons or they are seeing a different Doctor. Due to the limited space available and to maintain other patients’ privacy and dignity, we only allow patients (and carers) through to the ward area. Relatives/escorts will be contacted once the person is available for collection. The Endoscopy Unit endeavours to offer single sex facilities, and we aim to make your stay as comfortable and stress free as possible. -

Bariatric Surgery and Liver Transplantation
MAYO CLINIC Bariatric Surgery and Liver Transplantation Julie Heimbach, MD Professor and Chair, Transplantation Surgery Mayo Clinic, Rochester, MN [email protected] MAYO CLINIC Objectives • Outline current scope of the obesity epidemic • Implications of NASH pre and post LT • Discuss the role of bariatric surgery How can we best care for the obese liver transplant candidate? - World wide, obesity has doubled since 1980 - Currently, 600 million obese adults in the world MAYO CLINIC Why? • Clinical need for a different approach 4 MAYO CLINIC NASH as an indication for listing for liver transplantation in US Wong et al Gastro 2015; 148: 547-55. 5 MAYO CLINIC Why? • 57 year old male, BMI 52, MELD 30, referred to hospice by his local transplant center • LT+SG (MELD =40), current BMI=34 stable 3 years post LT • “One day I am dying, the next week I am not,” he said. “That just doesn’t happen.” 6 MAYO CLINIC Why? • Structured approach to the problem • Allows patients to return to full function– as transformative as transplant • Reduces the long-term complications of obesity 7 MAYO CLINIC Impact of obesity on pre-transplant patient selection • Most common cause of death for patients with NAFLD is a cardiovascular event. • Patients who undergo LT for NASH may be at an increased risk for perioperative/post-op cardiac events • Sarcopenia is associated with worse outcomes, including patients with sarcopenic obesity Ekstadt et al Hepatology 2006:4;865-73. Vanwagner et al Hepatology. 2012 Nov;56(5):1741-50 Choudary et al Clin Transplant 2015: 29: 211–215. -

Flexible Sigmoidoscopy – Outpatients
Flexible sigmoidoscopy – Outpatients You have been referred by your doctor to have a flexible sigmoidoscopy. his information booklet has been written to explain the procedure. This will help you to make an informed decision before consenting to the procedure. If you are unable to attend your appointment please inform us as soon as possible. Please ensure you read this booklet and the enclosed consent form thoroughly. Please also complete the enclosed health questionnaire. You may be contacted by an endoscopy trained nurse before your procedure to go through the admission process and answer any queries you may have. If you are not contacted please come to your appointment at the time stated in your letter. Please note your appointment time is your arrival time on the unit and not the time of your procedure. If you have any mobility issues or if there is a possibility you could be pregnant, please contact the appointment staff on 01284 713551 Please remember there will be other patients in the unit who may arrive after you but are taken in for their procedure before you, this is for medical reasons or they are seeing a different doctor. Due to limited space available and to maintain other patients’ privacy and dignity, we only allow patients (and carers) through to the ward area. Relatives/ escorts will be contacted once you are ready for collection. The Endoscopy Unit endeavours to offer single sex facilities, and we aim to make you stay as comfortable and stress free as possible. Source: Endoscopy Reference No: 5035-14 Issue date: 8/1/21 Review date: 8/1/24 Page 1 of 11 Medication If you are taking WARFARIN, CLOPIDOGREL, RIVAROXABAN or any other anticoagulant (blood thinning medication), please contact the appointment staff on 01284 713551, your GP or anticoagulation nurse, as special arrangements may be necessary. -

Intestinal and Multivisceral Transplant Reference Number: CP.MP.58 Coding Implications Last Review Date: 05/20 Revision Log
Clinical Policy: Intestinal and Multivisceral Transplant Reference Number: CP.MP.58 Coding Implications Last Review Date: 05/20 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Medical necessity criteria for the review of intestinal and multivisceral transplant requests. Policy/Criteria It is the policy of health plans affiliated with Centene Corporation® that any of the intestinal and/or multivisceral transplantation procedures listed in I are medically necessary for pediatric and adult members to restore function in those with irreversible intestinal failure when meeting the criteria in section II: I. Transplantation Procedures A. Isolated intestinal transplantation is indicated for members who have only isolated intestinal failure and no liver disease. B. Combined intestinal and liver transplant is indicated in those with intestinal failure and end stage liver disease. C. Multivisceral transplant is indicated in those with intestinal failure and gastrointestinal motility disorders (e.g., chronic idiopathic intestinal pseudo-obstruction, visceral myopathy, visceral neuropathy, total intestinal aganglionosis, and some forms of mitochondrial respiratory chain disorders that affect gastrointestinal motor function), or extensive mesenteric thrombosis. II. Procedure Criteria: Members must have one of the indications in A and none of the contraindications in B: A. Indications, any one of the following: 1. Failure of total parenteral nutrition (TPN) as indicated by one of the following: a. Impending or overt liver failure due to TPN, indicated by elevated serum bilirubin and/or liver enzymes, splenomegaly, thrombocytopenia, gastro-esophageal varices, coagulopathy, peristomal bleeding, or hepatic fibrosis/cirrhosis; b. Thrombosis of ≥ 2 central veins, including jugular, subclavian, and femoral veins; c. -

Anesthesiology Services for Gastrointestinal Endoscopy Reference Number: CP.MP.161 Coding Implications Last Review Date: 05/19 Revision Log
Clinical Policy: Anesthesiology Services for Gastrointestinal Endoscopy Reference Number: CP.MP.161 Coding Implications Last Review Date: 05/19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description Conscious sedation for gastrointestinal (GI) endoscopic procedures is standard of care to relieve patient anxiety and discomfort, improve outcomes of the examination, and decrease the memory of the procedure. A combination of an opioid and benzodiazepine is the recommended regimen for achieving minimal to moderate sedation for upper endoscopy and colonoscopy in people without risk for sedation-related adverse events.5 Generally, the gastroenterologist performing the procedure and/or his/her qualified assistant can adequately manage the administration of conscious sedation and monitoring of the patient. In cases with sedation-related risk factors, additional assistance from an anesthesia team member is required to ensure the safest outcome for the patient. This policy outlines the indications for which anesthesia services are considered medically necessary. Policy/Criteria I. It is the policy of health plans affiliated with Centene Corporation® that anesthesiology services for GI endoscopic procedures is considered medically necessary for the following indications: A. Age < 18 years or ≥ 70 years; B. Pregnancy; C. Increased risk of complications due to physiological status as identified by the American Society of Anesthesiologist (ASA) physical status classification of ASA III or higher; D. Increased risk for airway obstruction because of anatomic variants such as dysmorphic facial features, oral abnormalities, neck abnormalities, or jaw abnormalities; E. History of or anticipated intolerance to conscious sedation (i.e. chronic opioid or benzodiazepine use); F.