Summary of Product Characteristics 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hepatitis B Patient with Fever Due to Focal Infection
DOI: 10.5272/jimab.2012184.251 Journal of IMAB - Annual Proceeding (Scientific Papers) 2012, vol. 18, book 4 HEPATITIS B PATIENT WITH FEVER DUE TO FOCAL INFECTION Assya Krasteva*, Dobriana Panova**, Krasimir Antonov**, Angelina Kisselova* * Department of Imaging and Oral Diagnostic, Faculty of Dental Medicine, ** Clinic of gastroenterology, “St. Ivan Rilski” University Hospital, Medical University - Sofia, Bulgaria. ABSTRACT enzymes, CRP and albumin returned to normal after Persistent undiagnosed fever remains a common performance of dental recommendations. problem in clinical practice. It is a fact that dental sepsis Key words: fever, focal infection, dental sepsis is one potential cause of persistent fever that can escape detection (Siminoski, K., 1993). INTRODUCTION We present a 50 years old woman with chronic Adult patients frequently visit physicians with date hepatitis B, febrile for the past two months (max. 38.60C) of persistent fever and the cause of the fever sometimes with characteristic of septic fever. She underwent cannot be found. consultations with endocrinologist, rheumatologist, The definition of fever of unknown origin (FUO), as neurologist, gynecologist, pulmonologist and infectionst. All based on a case series of 100 patients, is defined as a negative for any disease, also negative serology for Lyme temperature higher than 38.3 C (100.9 F) that lasts for more disease. She had no data for urine infection. She had than three weeks with no obvious source despite appropriate negative cultures. The patient was treated with investigation (Roth, A., 2003), and evaluation of at least 3 corticosteroids for 30 days with no effect; she had no outpatient visits or 3 days in hospital (Durack, DT.,1991). -

Drug Prescribing for Dentistry Dental Clinical Guidance
Scottish Dental Clinical Effectiveness Programme SDcep Drug Prescribing For Dentistry Dental Clinical Guidance Second Edition August 2011 Scottish Dental Clinical Effectiveness Programme SDcep The Scottish Dental Clinical Effectiveness Programme (SDCEP) is an initiative of the National Dental Advisory Committee (NDAC) and is supported by the Scottish Government and NHS Education for Scotland. The programme aims to provide user-friendly, evidence-based guidance for the dental profession in Scotland. SDCEP guidance is designed to help the dental team provide improved care for patients by bringing together, in a structured manner, the best available information that is relevant to priority areas in dentistry, and presenting this information in a form that can interpreted easily and implemented. ‘Supporting the dental team to provide quality patient care’ Scottish Dental Clinical Effectiveness Programme SDcep Drug Prescribing For Dentistry Dental Clinical Guidance Second Edition August 2011 Drug Prescribing For Dentistry © Scottish Dental Clinical Effectiveness Programme SDCEP operates within NHS Education for Scotland. You may copy or reproduce the information in this document for use within NHS Scotland and for non-commercial educational purposes. Use of this document for commercial purpose is permitted only with written permission. ISBN 978 1 905829 13 2 First published 2008 Second edition published August 2011 Scottish Dental Clinical Effectiveness Programme Dundee Dental Education Centre, Frankland Building, Small’s Wynd, Dundee DD1 -

Clinical Characteristics and Outcomes of Paediatric Orbital Cellulitis in Hospital Universiti Sains Malaysia: a Five-Year Review
Singapore Med J 2020; 61(6): 312-319 Original Article https://doi.org/10.11622/smedj.2019121 Clinical characteristics and outcomes of paediatric orbital cellulitis in Hospital Universiti Sains Malaysia: a five-year review Ismail Mohd-Ilham1,2, MBBS, MMed, Abd Bari Muhd-Syafi1,2, MBBS, Sonny Teo Khairy-Shamel1,2, MD, MMed, Ismail Shatriah1,2, MD, MMed INTRODUCTION Limited data is available on paediatric orbital cellulitis in Asia. We aimed to describe demographic data, clinical presentation, predisposing factors, identified microorganisms, choice of antibiotics and management in children with orbital cellulitis treated in a tertiary care centre in Malaysia. METHODS A retrospective review was performed on children with orbital cellulitis aged below 18 years who were admitted to Hospital Universiti Sains Malaysia, Kelantan, Malaysia, between January 2013 and December 2017. RESULTS A total of 14 paediatric patients fulfilling the diagnostic criteria for orbital cellulitis were included. Their mean age was 6.5 ± 1.2 years. Boys were more likely to have orbital cellulitis than girls (71.4% vs. 28.6%). Involvement of both eyes was observed in 14.3% of the patients. Sinusitis (28.6%) and upper respiratory tract infection (21.4%) were the most common predisposing causes. Staphylococcus aureus (28.6%) was the leading pathogen. Longer duration of hospitalisation was observed in those infected with methicillin-resistant Staphylococcus aureus and Burkholderia pseudomallei. 10 (71.4%) patients were treated with a combination of two or three antibiotics. In this series, 42.9% had surgical interventions. CONCLUSION Young boys were found to be more commonly affected by orbital cellulitis than young girls. -

Patients Diagnosed with Infective Endocarditis: a Retrospective Chart Review
Patients Diagnosed with Infective Endocarditis: A Retrospective Chart Review Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Chloe A. Wong, DMD Graduate Program in Dentistry The Ohio State University 2020 Thesis Committee Ashok Kumar DDS, MS, Advisor Paul Casamassimo, DDS, MS Daniel Claman, DDS John Kovalchin, MD William Hunt, MD Copyrighted by Chloe A. Wong DMD 2020 2 Abstract Purpose: To describe characteristics and associations of risk factors for patients admitted to Nationwide Children’s Hospital (NCH) for infective endocarditis (IE), and to provide a descriptive overview of IE in a representative U.S. children’s hospital. Methods: A retrospective chart review of electronic medical records from January 1, 2008 to January 1, 2020 of patients who met the modified Duke criteria for definite or possible IE. Study variables include demographics, medical and cardiac history, predisposing conditions and risk factors, bacterial isolates, hospital course, treatment, complications, and dental history. Results: Initial search query found 242 patients. 67 patients met inclusion criteria. 69% had an underlying cardiac condition. S. aureus and viridans streptococcus were most common. Age was significantly associated with presence of intracardiac hardware. The mean length of hospital stay was 25 days and the mortality rate was 9%. 32% patients had a dental consult during admission. Conclusion: Increased survival of children with significant heart conditions increases the likelihood of them becoming pediatric dental patients. Occurrence of IE in healthy children and the questioning of IE association with dental procedures suggest further research is needed. -

The Diagnosis & Management of Skin & Soft Tissue Infection
THE DIAGNOSIS & MANAGEMENT OF SKIN & SOFT TISSUE INFECTION 7th December 2020 7th December 2022 Version History Version Status Date Editor Description 1.0 Final 7th December 2020 Guidelines Team Version for Publication. Citation Suggested citation style: Ministry of Public Health Qatar. National Clinical Guideline: The Diagnosis and Management of Skin and Soft Tissue Infection (2020). Abbreviations The abbreviations used in this guideline are as follows: ART Antiretroviral Therapy BIT Burrow Ink Test CA-MRSA Community-Associated Methicillin-Resistant Staphylococcus aureus CT Computed Tomography EPSD Epidermal Parasitic Skin Diseases FGSI Fournier’s Gangrene Severity Index HIV Human Immunodeficiency Virus HrCLM Hookworm-Related Cutaneous Larva Migrans LRINEC Laboratory Risk Indicator for Necrotising Fasciitis MMR Measles-Mumps-Rubella MCV Molluscum Contagiosum Virus MMRV Measles-Mumps-Rubella-Varicella MRI Magnetic Resonance Imaging MRSA Methicillin-Resistant Staphylococcus aureus MSSA Methicillin-Sensitive Staphylococcus aureus NSAID Non-Steroidal Anti-Inflammatory Drug S. aureus Staphylococcus aureus S. pyogenes Streptococcus pyogenes SSTIs Skin and Soft-Tissue Infections VZV Varicella Zoster Virus The Diagnosis and Management of Skin and Soft Tissue Infection (Date of next revision: 7th December 2022) 2 Table of Contents 1 Information about this Guideline ........................................................................................................... 6 1.1 Objective and Purpose of the Guideline ....................................................................................... -

Overview of Fever of Unknown Origin in Adult and Paediatric Patients L
Overview of fever of unknown origin in adult and paediatric patients L. Attard1, M. Tadolini1, D.U. De Rose2, M. Cattalini2 1Infectious Diseases Unit, Department ABSTRACT been proposed, including removing the of Medical and Surgical Sciences, Alma Fever of unknown origin (FUO) can requirement for in-hospital evaluation Mater Studiorum University of Bologna; be caused by a wide group of dis- due to an increased sophistication of 2Paediatric Clinic, University of Brescia eases, and can include both benign outpatient evaluation. Expansion of the and ASST Spedali Civili di Brescia, Italy. and serious conditions. Since the first definition has also been suggested to Luciano Attard, MD definition of FUO in the early 1960s, include sub-categories of FUO. In par- Marina Tadolini, MD Domenico Umberto De Rose, MD several updates to the definition, di- ticular, in 1991 Durak and Street re-de- Marco Cattalini, MD agnostic and therapeutic approaches fined FUO into four categories: classic Please address correspondence to: have been proposed. This review out- FUO; nosocomial FUO; neutropenic Marina Tadolini, MD, lines a case report of an elderly Ital- FUO; and human immunodeficiency Via Massarenti 11, ian male patient with high fever and virus (HIV)-associated FUO, and pro- 40138 Bologna, Italy. migrating arthralgia who underwent posed three outpatient visits and re- E-mail: [email protected] many procedures and treatments before lated investigations as an alternative to Received on November 27, 2017, accepted a final diagnosis of Adult-onset Still’s “1 week of hospitalisation” (5). on December, 7, 2017. disease was achieved. This case report In 1997, Arnow and Flaherty updated Clin Exp Rheumatol 2018; 36 (Suppl. -

Dental Abscess: Primary and Permanent Teeth ______Ears, Eyes, Nose, Throat and Mouth
Dental Abscess: Primary and Permanent Teeth _____________________________ Ears, Eyes, Nose, Throat and Mouth Clinical Decision Tool for RNs with Effective Date: December 1, 2019 Authorized Practice [RN(AAP)s] Review Date: December 1, 2022 Background Dental abscess is an acute infection of the teeth or the structures supporting the teeth and/or gums. Twenty primary teeth typically erupt between six months and two years of age (Stephens, Wiedemer, & Kushner, 2018). The typical adult has 32 permanent teeth which begin to erupt at about age six with the process complete by age 18 (Stephens et al., 2018). Dental abscesses are the result of untreated dental caries (Stephens et al., 2018). There are two types of dental abscess including apical and periodontal (Robertson, Keys, Rautemaa-Richardson, Burns, & Smith, 2015). Apical abscesses are the most common and originate in the dental pulp (Robertson et al., 2015). Periodontal abscesses originate in the supporting structure of the teeth, between teeth and gums, and are usually a result of chronic periodontitis (Robertson et al., 2015). Most commonly dental abscesses are caused by a combination of oral streptococci (especially the Streptococcus anginosus group), and strict anaerobes (such as anaerobic streptococci, Prevotella species, and Fusobacterium species) (Robertson et al., 2015; Troeltzsch et al., 2015). Immediate Consultation Requirements The RN(AAP) should seek immediate consultation from a physician/NP when any of the following circumstances exist: ● gingival or facial cellulitis -

Fever of Unknown Origin (FUO) Clinical Presentation Updated: Mar
Fever of Unknown Origin (FUO) Clinical Presentation Updated: Mar 20, 2017 Author: Sandra G Gompf, MD, FACP, FIDSA; Chief Editor: Michael Stuart Bronze, MD Sandra G Gompf, MD, FACP, FIDSA Associate Professor of Infectious Diseases and International Medicine, University of South Florida College of Medicine; Chief, Infectious Diseases Section, Director, Occupational Health and Infection Control Programs, James A Haley Veterans Hospital Sandra G Gompf, MD, FACP, FIDSA is a member of the following medical societies: American College of Physicians, Infectious Diseases Society of America Specialty Editor Board Francisco Talavera, PharmD, PhD Adjunct Assistant Professor, University of Nebraska Medical Center College of Pharmacy; Editor-in-Chief, Medscape Drug Reference Disclosure: Received salary from Medscape for employment. for: Medscape. Charles V Sanders, MD Edgar Hull Professor and Chairman, Department of Internal Medicine, Professor of Microbiology, Immunology and Parasitology, Louisiana State University School of Medicine at New Orleans; Medical Director, Medicine Hospital Center, Charity Hospital and Medical Center of Louisiana at New Orleans; Consulting Staff, Ochsner Medical Center Charles V Sanders, MD is a member of the following medical societies: American College of Physicians, Alliance for the Prudent Use of Antibiotics, The Foundation for AIDS Research, Southern Society for Clinical Investigation, Southwestern Association of Clinical Microbiology, Association of Professors of Medicine, Association for Professionals in Infection -
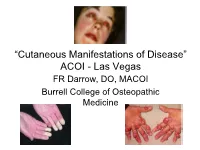
Dermatological Indications of Disease - Part II This Patient on Dialysis Is Showing: A
“Cutaneous Manifestations of Disease” ACOI - Las Vegas FR Darrow, DO, MACOI Burrell College of Osteopathic Medicine This 56 year old man has a history of headaches, jaw claudication and recent onset of blindness in his left eye. Sed rate is 110. He has: A. Ergot poisoning. B. Cholesterol emboli. C. Temporal arteritis. D. Scleroderma. E. Mucormycosis. Varicella associated. GCA complex = Cranial arteritis; Aortic arch syndrome; Fever/wasting syndrome (FUO); Polymyalgia rheumatica. This patient missed his vaccine due at age: A. 45 B. 50 C. 55 D. 60 E. 65 He must see a (an): A. neurologist. B. opthalmologist. C. cardiologist. D. gastroenterologist. E. surgeon. Medscape This 60 y/o male patient would most likely have which of the following as a pathogen? A. Pseudomonas B. Group B streptococcus* C. Listeria D. Pneumococcus E. Staphylococcus epidermidis This skin condition, erysipelas, may rarely lead to septicemia, thrombophlebitis, septic arthritis, osteomyelitis, and endocarditis. Involves the lymphatics with scarring and chronic lymphedema. *more likely pyogenes/beta hemolytic Streptococcus This patient is susceptible to: A. psoriasis. B. rheumatic fever. C. vasculitis. D. Celiac disease E. membranoproliferative glomerulonephritis. Also susceptible to PSGN and scarlet fever and reactive arthritis. Culture if MRSA suspected. This patient has antithyroid antibodies. This is: • A. alopecia areata. • B. psoriasis. • C. tinea. • D. lichen planus. • E. syphilis. Search for Hashimoto’s or Addison’s or other B8, Q2, Q3, DRB1, DR3, DR4, DR8 diseases. This patient who works in the electronics industry presents with paresthesias, abdominal pain, fingernail changes, and the below findings. He may well have poisoning from : A. lead. B. -

South Central Antimicrobial Network Guidelines for Antibiotic Prescribing in the Community 2018
South Central Antimicrobial Network Guidelines for Antibiotic Prescribing in the Community 2018 Adapted from the Public Health England (PHE) and British Infection Association Management of Infection Guidance for Primary Care by the South Central Antimicrobial Network Group (SCAN) In conjunction with all WESSEX CCGs, Berkshire East, Berkshire West, Surrey Heath, Coastal West Sussex and Oxfordshire CCG Index Foreword 3 n Recurrent UTI in Non-Pregnant n Threadworms 50 Guideline Development Group 5 Women – Prophylaxis 29 n Cholecystitis 51 Aims, Implications, Principles 7 n Acute Pyelonephritis (Upper UTI) 30 Skin & Soft tissue Infections Risk Assessment 9 Genital Tract Conditions n Impetigo 53 Sepsis Screening 11 n Criteria for referring patients to n Scabies 54 Ear, Nose and Throat Infections specialist care 31 n Eczema 55 n Acute Sore Throat 13 n Vulvovaginal Candidiasis 32 n Acne Vulgaris 56 n Fever Pain 14 n Bacterial Vaginosis 33 n Acne Rosacea 57 n Acute Otitis Media (AOM) 15 n Chlamydia Trachomatis 34 n Cellulitis 58 n Acute Otitis Externa 16 n Trichomoniasis 35 n Leg Ulcers 59 n Acute Rhinosinusitis 17 n Pelvic Inflammatory Disease (PID) 36 n Diabetic Foot Ulcer 60 n Oral Candidiasis 18 n Acute Prostatitis 37 n MRSA (meticillin-resistant n Balanitis 38 Staphylococcus aureus) 61 Respiratory Tract Infections n Epididymo-Orchitis 39 n Animal Bite 62 n Acute Cough, Bronchitis 19 n Genital Herpes 40 n Human Bite 63 n Influenza 20 n Insect bites 64 n Bartholin’s Cyst, Post Top n COPD Acute Exacerbation 21 Endometritis, Gonorrhoea 41 n Fungal Infection – Skin 65 n Community-Acquired Pneumonia (CAP) 22 n Fungal Infection – Fingernail or Toenail 66 Gastro-intestinal Infections Central Nervous System n Varicella Zoster (chicken pox), n Eradication of Helicobacter pylori 43 n Meningitis or Suspected Herpes Zoster (shingles) & Cold Sores 67 n Eradication of Helicobacter pylori cont. -

Dental Abscess
6/14/2016 Dental Abscess HumanResearchWiki Dental Abscess From HumanResearchWiki Contents 1 Introduction 2 Clinical Priority and Clinical Priority Rationale by Design Reference Mission 3 Initial Treatment Steps During Space Flight 4 Capabilities Needed for Diagnosis 5 Capabilities Needed for Treatment 6 Associated Gap Reports 7 Other Pertinent Documents 8 List of Acronyms 9 References 10 Last Update Introduction The apical foramen is an opening located at the apex of a tooth through which nerves and blood vessels traverse, forming the pulp. An acute apical abscess manifests with pain and possibly a purulent exudate (pus) around the apex of a tooth. It is the result of an acute periapical periodontitis, which itself results from pulpal necrosis and extensive inflammation.[1] The most common symptom is pain but can also include sensitivities to temperature, pressure, and percussion. A periapical abscess elevates the tooth from its socket and feels “high” when the patient bites down.[2] Disease progression is associated with an increasing severity and duration of pain. Clinical Priority and Clinical Priority Rationale by Design Reference Mission One of the inherent properties of space flight is a limitation in available mass, power, and volume within the space craft. These limitations mandate prioritization of what medical equipment and consumables are manifested for the flight, and which medical conditions would be addressed. Therefore, clinical priorities have been assigned to describe which medical conditions will be allocated resources for diagnosis and treatment. “Shall” conditions are those for which diagnostic and treatment capability must be provided, due to a high likelihood of their occurrence and severe consequence if the condition were to occur and no treatment was available. -

Investigation of Pus and Exudates
UK Standards for Microbiology Investigations Investigation of pus and exudates Issued by the Standards Unit, Microbiology Services, PHE Bacteriology | B 14 | Issue no: 6.2 | Issue date: 22.11.16 | Page: 1 of 34 © Crown copyright 2016 Investigation of pus and exudates Acknowledgments UK Standards for Microbiology Investigations (SMIs) are developed under the auspices of Public Health England (PHE) working in partnership with the National Health Service (NHS), Public Health Wales and with the professional organisations whose logos are displayed below and listed on the website https://www.gov.uk/uk- standards-for-microbiology-investigations-smi-quality-and-consistency-in-clinical- laboratories. SMIs are developed, reviewed and revised by various working groups which are overseen by a steering committee (see https://www.gov.uk/government/groups/standards-for-microbiology-investigations- steering-committee). The contributions of many individuals in clinical, specialist and reference laboratories who have provided information and comments during the development of this document are acknowledged. We are grateful to the medical editors for editing the medical content. For further information please contact us at: Standards Unit Microbiology Services Public Health England 61 Colindale Avenue London NW9 5EQ E-mail: [email protected] Website: https://www.gov.uk/uk-standards-for-microbiology-investigations-smi-quality- and-consistency-in-clinical-laboratories PHE publications gateway number: 2016127 UK Standards for Microbiology Investigations are produced in association with: Logos correct at time of publishing. Bacteriology | B 14 | Issue no: 6.2 | Issue date: 22.11.16 | Page: 2 of 34 UK Standards for Microbiology Investigations | Issued by the Standards Unit, Public Health England Investigation of pus and exudates Contents Acknowledgments ................................................................................................................