2020-08 Ccts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
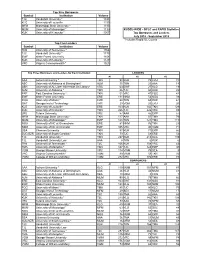
Symbol Institution Volume TJC Vanderbilt University * 1338 KLG
Top Five Borrowers Symbol Institution Volume TJC Vanderbilt University * 1338 KLG University of Louisville 1239 MFM Mississippi State University * 1193 MUM University of Mississippi * 1139 KUDZU-WIDE - OCLC and RAPID Statistics KUK University of Kentucky * 1057 Top Borrowers and Lenders July 2018 - September 2018 * Includes Rapid ILL Counts Top Five Lenders Symbol Institution Volume TKN University of Tennessee * 1764 TJC Vanderbilt University * 1175 EWF Wake Forest University 1154 KUK University of Kentucky * 1127 VRC Virginia Commonwealth * 1021 Top Three Borrowers and Lenders for Each Institution LENDERS #1 #2 #3 AAA Auburn University * TKN 98 KUK 75 LRU 74 ABC University of Alabama at Birmingham* ALM 35 TKN 23 AAA 18 ABH University of AL Lister Hill Health Sci Library* VRC 63 EWF 27 KLG 19 ALM University of Alabama * TKN 86 TJC 60 KUK 49 ERE East Carolina University * TKN 51 VRC 47 FQG 42 EWF Wake Forest University ERE 131 NKM 54 NGU 38 FQG University of Miami * EWF 80 TKN 65 KUK 61 GAT Georgia Inst of Technology VRC 29 VGM 23 LRU 20 KLG University of Louisville ERE 163 KUK 142 TKN 129 KUK University of Kentucky * TKN 266 TJC 226 VRC 93 LRU Tulane University VRC 87 AAA 85 EWF 57 MFM Mississippi State University * TKN 117 AAA 87 TMA 79 MUM University of Mississippi * EWF 128 TKN 121 TMA 115 NGU University of NC at Greensboro ERE 81 NKM 76 TKN 23 NKM University of NC at Charlotte EWF 105 VRC 90 VGM 71 SEA Clemson University TKN 97 KUK 71 EWF 66 SUC/SZR University of South Carolina * TKN 73 TJC 58 ERE 54 TJC Vanderbilt University * TKN -

2018-19 New Faculty Booklet
New Faculty 2018–2019 Dear Colleagues and Friends, The new faculty members we celebrate in this publication join an institution that has pro- vided more than 175 years of scholarship and service to its community. Founded in 1834 as the Medical College of Louisiana to address public health concerns in the region, the Tulane University of today is recognized as one of the nation’s preeminent research universities, carrying out pathbreaking research and creative work, offering an unparalleled education to a cohort of outstanding undergraduate, graduate and professional students, and earning recognition as a national and world leader in public service and social engagement. Tulane professors have been awarded the most prestigious honors in the academic world, and have been elected to membership in the National Academies and in the American Academy for Arts and Sciences. In the past 35 years alone, Tulane colleagues have received 25 Fulbright Fellowships, 11 National Science Foundation CAREER Awards, nine Guggenheim Foundation Fellowships, eight National Endowment for the Humanities Fellowships, four Alfred P. Sloan Fellowships and two Nobel Prizes in Medicine. Our schools and our undergraduate college offer an impressive array of degrees in architecture, business, law, liberal arts, medicine, public health and tropical medicine, science and engineering, and social work. Interdisciplinary research is flourish- ing, as our students and faculty are crossing both geographic and intellectual boundaries in their efforts to ask new questions, create new knowledge and improve the lives of people around the world. Each year we recruit to Tulane some of the smartest and most talented students in the world, attracted to Tulane because of their interest in a demanding, rigorous education, and in the opportunities that will be open to them when they complete their study and are awarded a degree from one of the most recognized and respected universities in the world. -

University.Pdf
2021-2022 1 THE UNIVERSITY Mission Statement Tulane’s purpose is to create, communicate and conserve knowledge in order to enrich the capacity of individuals, organizations, and communities to think, to learn, and to act and lead with integrity and wisdom. Tulane pursues this mission by cultivating an environment that focuses on learning and the generation of new knowledge; by expecting and rewarding teaching and research of extraordinarily high quality and impact; and by fostering community-building initiatives as well as scientific, cultural and social understanding that integrate with and strengthen learning and research. This mission is pursued in the context of the unique qualities of our location in New Orleans and our continual aspiration to be a truly distinctive international university. History Tulane University, one of the foremost independent national research universities in the country, is ranked among the top quartile of the nation’s most highly selective universities. With ten schools and colleges that range from the liberal arts and sciences through a full spectrum of professional schools, Tulane gives its students a breadth of choice equaled by few other independent universities in the country. Tulane University’s ten academic divisions enroll approximately 8,000 undergraduates and about 5,000 graduate and professional students. The schools of Architecture, Business, Liberal Arts, Public Health and Tropical Medicine, and Science and Engineering offer both undergraduate and graduate programs. Other divisions include the schools of Law, Medicine, Social Work and Professional Advancement. Tulane traces it origins back to the founding of the Medical College of Louisiana, the Deep South’s first medical school, in 1834. -

ASEP on Campus
ASEP on Campus Colleges and universities that offer ASEP’s Coaching Principles or Sport First Aid Alabama D.C. Iowa Athens State University George Washington University Cornell College University of Alabama–Birmingham Florida Grinnell College US Sports Academy Florida Memorial College Iowa State University Florida State University Iowa Western Community College Alaska Scott Community College University of Alaska–Anchorage Indian River Community College Palm Beach Atlantic University University of Northern Iowa Waldorf College Arizona Stetson University Western Iowa Tech Community College Prima County Community College University of Florida University of South Florida Kansas Arkansas University of Tampa Kansas Wesleyan Arkansas Tech University Warner Southern College Ottawa University Central Baptist College Georgia California Louisiana Brewton Parker College Cal State–Chico Nicholls State University Georgia College & State University Cal State–East Bay Georgia Southern University Cal State–Fresno Maine Kennesaw State University Cal State–Fullerton Bates College Cal State–Northridge Husson College Idaho Northern Maine Technical College Cal Poly State University Boise State University Concordia College Thomas College Brigham Young–Idaho University of Maine Fresno Pacific University University of Idaho Modesto Junior College University of Maine at Presqe Isle Ohlone College Illinois Maryland San Diego Christian College Blackburn College Columbia Union College San Diego State University College of DuPage Morgan State University Concordia University Salisbury State University Colorado Elmhurst College Colorado State University Greenville Christian College Massachusetts Ft. Lewis College Heartland Community College College of Our Lady of the ELMS Northeastern Junior College Illinois State University Smith College John Wood Community College Connecticut Western New England College North Central College Gallaudet University Triton College Michigan St. -

TULANE Vs. ALABAMA Lute~E Ta Uti ME OUT" with Johnny Lynch WWL Thursdays 9:45 to 10 P.M • • Uworld of SPORTS"
TULANE vs. ALABAMA Lute~e ta uTI ME OUT" with Johnny Lynch WWL Thursdays 9:45 to 10 P.M • • uWORLD OF SPORTS". with Bill Brengel JACKSON BREWING CO. WWL thru Sat. 5:35 5:45 P.M. NEW ORLEANS. LA. Mon. to Tulane Stadium Vol. 18 T H E - c;·~· R EE N I E No. Official Souvenir F oot/,all Program of Tulane University CONTENTS Page Editorial . 3 The Presidents . 5 Tulane Yells . 6 Tulane Roster . 7 Cam-Pix . ............ 9-12, 17-20 National Starting Lineups . .... 14-15 Advertising SEC Schedules . 21 Representatives Alabama Roster ......... 22-23 Football Publi Co-Editors, This is Alabama .. .... .. 2!:: cations, Inc Tulane Songs . 26 370 Lexington A venue ANDY RoGERS Pigskin Roundup 27 New York, N. Y. BILL ]8HNSTON Football Ticket ••• plus your MB Label-of-Quality! Now. as always. Maison Blanche has the line-up of the famous labels you want and buy with confidence. No matter what the occasion ••• MB has the right clothes • • • on one of its famous fashion floors . • MAISON BLANCIIE GREATEST STORE SOUTH 2 IT'S THAT TIME AGAIN Lay aside your baseball bats, store that classed as "perhaps too light for this flannel in moth balls, swap those low quar league," But what they may lack in ter shoes for high tops-it's football time weight, Frnka believes, may be added in again! · that synonym for the 20th century- speed. Yes sir, you may read about the World Veterans of many a Green Wave battle Series and the number of home runs by will be back-Seniors like Emile O'Brien Joe DiMaggio and the records set during and Don Fortier, juniors such as speed the past baseball season. -

Curriculum Vitae Gloria T Lessan
Curriculum Vitae Gloria T Lessan February 05, 2020 General Information University address: Sociology College of Social Sciences and Public Policy Bellamy Building 0526 Florida State University Tallahassee, Florida 32306-2270 Phone: 850-644-6416; Fax: 850-644-6208 E-mail address: [email protected] Professional Preparation 1987 Doctor of Philosophy, Tulane University of Louisiana. Major: Sociology. 1980 Master of Art, University of Wisconsin at Milwaukee. Major: Sociology. 1972 Bachelor of Science, Universidad Autonoma de Nuevo Leon, Mexico. Major: Industrial Engineering. Nondegree Education and Training 2016 Global Partner Certificate. Professional Experience 2017–2018 Teaching (Level III) Professor, Florida State University. 2012–2017 Associate-In-Teaching, Florida State University, Florida State University. 2012 Assistant-In-Teaching, Florida State University. 2008–2012 Lecturer, Sociology, Florida State University. 2005–2008 Visiting Lecturer, Sociology, Florida State University. 2001–2005 Adjunct Instructor, Sociology, Florida State University. Vita for Gloria T Lessan 1997–2002 Adjunct Professor, Social Sciences and History Division, Tallahassee Community College. 1996 Secretary Specialist, Office of Modernization, FLDEP/Division of State Lands. 1994 Adjunct Professor, School of Criminology, Florida State University. 1987–1991 Assistant Professor, Department of Sociology/Criminal Justice, Old Dominion University. 1986–1987 Visiting Instructor, Department of Sociology, Tulane University. 1983–1986 Teaching Assistant, Department of Sociology, Tulane University. 1983–1984 Research Analysts, Orleans Parish Sheriff's Office, New Orleans, LA. (Development of a statistical profile of inmates for purposes of classification and statistical profile of loyal employee). 1979–1980 Research Assistant, Department of Sociology, University of Wisconsin-Milwaukee. 1975–1978 Faculty, Department of Behavioral Sciences, ITESM, Monterrey, N.L. Mexico. Honors, Awards, and Prizes 2018-2019 Graduate Student Mentorship Award, Department of Sociology (2019). -

Curriculum Vitae
NEETI NAIR Corcoran Department of History University of Virginia P. O. Box 400180 Charlottesville, VA 22904 email: [email protected] ACADEMIC EMPLOYMENT University of Virginia, Corcoran Department of History, Associate Professor, fall 2012 - present University of Virginia, Corcoran Department of History, Assistant Professor, 2006 - 2012 University of Virginia, South Asia Center, Core Faculty, 2006 - 2016 Tulane University, Department of History, Assistant Professor, spring 2006 Brown University, Department of History, Visiting Instructor, spring 2005, spring 2004 EDUCATION Ph.D. in History. Tufts University, 2005 M.A. in History. Tufts University, 2000 B.A. in History (Honours). St. Stephen’s College, University of Delhi. First Class. 1998 Indian School Certificate. Rishi Valley School, Krishnamurti Foundation India. 1995 PUBLICATIONS BOOKS Changing Homelands: Hindu Politics and the Partition of India, Cambridge, MA: Harvard University Press 2011, 356 pages including notes, bibliography Co-published by Permanent Black in India, 2011; Paperback, 2016 Short-listed for the AHA’s inaugural John F. Richards Prize for South Asian History, 2011 A Washington Post WorldViews Recommended Book, 2013 Invited essays on related themes: Page 99 Test, India Today, Seminar Reviews: The American Historical Review, Contemporary South Asia, Indian Economic and Social History Review, Journal of Asian Studies, South Asia: Journal of South Asian Studies, Journal of the Economic and Social History of the Orient, Social History, Asian Affairs, 1 Journal of Genocide -

College Acceptances
UPPER SCHOOL CLASS OF 2020 College Acceptances Adelphi University Lehigh University American University LIM College (Laboratory Arizona State University Institute of Merchandising) Auburn University Louisiana State University Babson College Loyola University Chicago Barry University Loyola University New Orleans Baruch College of the CUNY Lynn University Bentley University McGill University Binghamton University Michigan State University Boston College Millikin University Boston University Muhlenberg College Brandeis University New College of Florida Broward College New School Brown University New York University California Institute of Technology North Carolina State University California Polytechnic State University, Northeastern University San Luis Obispo Northwestern University California State University-San Bernardino Nova Southeastern University University of Illinois Carnegie Mellon University Ohio State University University of Kansas Case Western University Pace University University of Maryland Chapman University Pennsylvania State University University of Massachusetts Clemson University Pepperdine University University of Miami College of Charleston Piedmont College University of Michigan Colorado State University Point Park University University of Minnesota Columbia College Chicago Purdue University University of Mississippi Cornell University Rice University University of Missouri Davidson College Ringling College of Art and Design University of North Carolina at Asheville Drew University Rollins College University of -

FICE Code List for Colleges and Universities (X0011)
FICE Code List For Colleges And Universities ALABAMA ALASKA 001002 ALABAMA A & M 001061 ALASKA PACIFIC UNIVERSITY 001005 ALABAMA STATE UNIVERSITY 066659 PRINCE WILLIAM SOUND C.C. 001008 ATHENS STATE UNIVERSITY 011462 U OF ALASKA ANCHORAGE 008310 AUBURN U-MONTGOMERY 001063 U OF ALASKA FAIRBANKS 001009 AUBURN UNIVERSITY MAIN 001065 UNIV OF ALASKA SOUTHEAST 005733 BEVILL STATE C.C. 001012 BIRMINGHAM SOUTHERN COLL ARIZONA 001030 BISHOP STATE COMM COLLEGE 001081 ARIZONA STATE UNIV MAIN 001013 CALHOUN COMMUNITY COLLEGE 066935 ARIZONA STATE UNIV WEST 001007 CENTRAL ALABAMA COMM COLL 001071 ARIZONA WESTERN COLLEGE 002602 CHATTAHOOCHEE VALLEY 001072 COCHISE COLLEGE 012182 CHATTAHOOCHEE VALLEY 031004 COCONINO COUNTY COMM COLL 012308 COMM COLLEGE OF THE A.F. 008322 DEVRY UNIVERSITY 001015 ENTERPRISE STATE JR COLL 008246 DINE COLLEGE 001003 FAULKNER UNIVERSITY 008303 GATEWAY COMMUNITY COLLEGE 005699 G.WALLACE ST CC-SELMA 001076 GLENDALE COMMUNITY COLL 001017 GADSDEN STATE COMM COLL 001074 GRAND CANYON UNIVERSITY 001019 HUNTINGDON COLLEGE 001077 MESA COMMUNITY COLLEGE 001020 JACKSONVILLE STATE UNIV 011864 MOHAVE COMMUNITY COLLEGE 001021 JEFFERSON DAVIS COMM COLL 001082 NORTHERN ARIZONA UNIV 001022 JEFFERSON STATE COMM COLL 011862 NORTHLAND PIONEER COLLEGE 001023 JUDSON COLLEGE 026236 PARADISE VALLEY COMM COLL 001059 LAWSON STATE COMM COLLEGE 001078 PHOENIX COLLEGE 001026 MARION MILITARY INSTITUTE 007266 PIMA COUNTY COMMUNITY COL 001028 MILES COLLEGE 020653 PRESCOTT COLLEGE 001031 NORTHEAST ALABAMA COMM CO 021775 RIO SALADO COMMUNITY COLL 005697 NORTHWEST -

Elliott Isaac
ELLIOTT ISAAC Tulane University Phone: (504) 862-8346 Department of Economics Email: [email protected] Tilton Hall, Room 203 Website: elliottisaac.com 6823 Saint Charles Avenue Citizenship: USA New Orleans, LA 70118 EMPLOYMENT AND AFFILIATIONS: Assistant Professor of Economics, Tulane University July 2018 – present Newcomb College Institute Faculty Fellow, Tulane University 2019 – present EDUCATION: Ph.D., Economics, University of Virginia May 2018 Dissertation: “The Tax Treatment of Marriage and its Impact on Family Formation and Labor Supply” Committee: Leora Friedberg (chair), Amalia Miller, Jonathan Colmer M.A., Economics, University of Virginia December 2013 B.A., Economics with Honors, Wake Forest University May 2011 FIELDS OF INTEREST: Public Economics, Labor Economics, Applied Microeconomics WORKING PAPERS: “Suddenly Married: Joint Taxation and the Labor Supply of Same-Sex Married Couples After U.S. v. Windsor” “Marriage, Divorce, and Tax and Transfer Policy” (Revise and resubmit at the Southern Economic Journal) “Elite Schools and Opting-In: Effects of College Selectivity on Career and Family Outcomes” with Suqin Ge and Amalia Miller “Same-Sex Marriage Recognition and Taxes: New Evidence about the Impact of Household Taxation” with Leora Friedberg (Submitted) “Spousal Bargaining Power: Decoupling Gender Norms and Earning Status” WORKS IN PROGRESS: “The Impact of Outreach on Delinquent Tax Filers” with James Alm, Matthias Kasper, and Erich Kirchler “Earnings Responses to Joint Taxation: Evidence from Same-Sex Couples” with James -

Colleges & Universities
Bishop Watterson High School Students Have Been Accepted at These Colleges and Universities Art Institute of Chicago Fordham University Adrian College University of Cincinnati Franciscan University of Steubenville University of Akron Cincinnati Art Institute Franklin and Marshall College University of Alabama The Citadel Franklin University Albion College Claremont McKenna College Furman University Albertus Magnus College Clemson University Gannon University Allegheny College Cleveland Inst. Of Art George Mason University Alma College Cleveland State University George Washington University American Academy of Dramatic Arts Coastal Carolina University Georgetown University American University College of Charleston Georgia Southern University Amherst College University of Colorado at Boulder Georgia Institute of Technology Anderson University (IN) Colorado College University of Georgia Antioch College Colorado State University Gettysburg College Arizona State University Colorado School of Mines Goshen College University of Arizona Columbia College (Chicago) Grinnell College (IA) University of Arkansas Columbia University Hampshire College (MA) Art Academy of Cincinnati Columbus College of Art & Design Hamilton College The Art Institute of California-Hollywood Columbus State Community College Hampton University Ashland University Converse College (SC) Hanover College (IN) Assumption College Cornell University Hamilton College Augustana College Creighton University Harvard University Aurora University University of the Cumberlands Haverford -

Southern Section C L a S S I C a L a S S O C I a T I O N O F T H E M I D D L E W
Southern Section Classical Association of the Middle West and South Nashville, Tennessee November 27-29, 1958 REVISED PROGRAM Officers President GRAYDON W. REGENOS, Tulane University Vice-President ISABEL JOHNSTON, Murrah H. S., Jackson, Miss. Secretary-Treasurer. ARTHUR F. STOCKER, Univ. of Virginia Elected Member of the Executive Committee THE REV. PAUL L. CALLENS, S. J., St. Charles College Local Committee on Arrangements H. Lloyd Stow, Vanderbilt University, chairman; Virginia Chaney, Belmont Col lege; Ann Dembsky, West End High School; Mrs. Paul McKnight, Belmont Col lege; Francis Newton, Vanderbilt University; Margaret H. Ottarson, The Harpeth Hall School; Mr. and Mrs. O. C. Peery, Peabody Demonstration School; Frederica Rawls, Hillsboro High School; Mary Walker, Isaac Litton High School Headquarters Alumni Hall Vanderbilt University Thursday 4. The Two-Year High School Latin Course (15 minutes) 27 November 1958 Ann Dembsky, West End High School, Nash ville, Tennessee 9:00 a.m. Registration, Memorial Room, Alumni Hall, Vanderbilt University. (To help defray 3:00 p.m. Bus tour of Nashville, led by Dr. A. L. the expenses of the meeting, a registration fee Crabb. Reservations for this tour should be of $i.oo will be charged.) made at the time of registration. 8:30 p.m. Third Session, Memorial Room, Alumni 9:45 a.m. First Session, Memorial Room, Alumni Hall, Vanderbilt University. Hall, Vanderbilt University. Alfred P. Hamilton, Millsaps College, presid H. Lloyd Stow, Vanderbilt University, presid ing. ing. Illustrated Lecture, "The Origin and Develop 1. The Bellum Civile of Petronius (20 minutes) ment of Humanistic Script." Robert E. Wolverton, University of Georgia B.