WJE Primer: Galvanic Corrosion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Corrosion Protection of Steel
Issue 73 February 2018 Corrosion Protection of Steel Introduction commercially available galvanized coatings used for cold-formed Carbon steel is widely used in all aspects of building construction steel framing members. due to its low cost, high strength and ease of fabrication. Corrosion is an inevitable phenomena that must be controlled to prolong the life of carbon steel components. Corrosion Corrosion is the natural process of the iron in steel combining with oxygen to form iron oxide. Corrosion occurs when steel is exposed to oxygen and water, which may be in the form of humid air. There are a number of factors that affect the rate of corrosion including the Figure 1: Zinc Coatings Weights and Thickness¹ composition of the steel alloy and environmental conditions (e.g. temperature, humidity, salinity, pH, pollution). If the zinc coating is damaged during fabrication or installation the area should be coated with zinc-rich paint or another accepted Corrosion is accelerated when carbon steel is in contact with a more repair method. cathodic metal, such as copper or stainless steel. Corrosion is detrimental for a number of reasons including: Interior Applications The risk of corrosion should be assessed and galvanized steel used • Corrosion weakens an item by replacing high-strength steel accordingly. The expected corrosion rate in most building interiors with lower-strength iron oxide, thereby reducing the effective area and cross section. is relatively low due to the controlled environment. Many laboratory • Iron oxide occupies greater volume than steel, so steel areas, however, are subject to high humidity, exposure to water and expands as it corrodes. -

GALVANIC/DISSIMILAR METAL CORROSION What It Is and How to Avoid It ASSDA Technical FAQ No 1 1 Edition 2, May 2009
AUSTRALIAN STAINLESS STEEL DEVELOPMENT ASSOCIATION GALVANIC/DISSIMILAR METAL CORROSION What it is and how to avoid it ASSDA Technical FAQ No 1 1 Edition 2, May 2009 Contact between dissimilar metals Metal to metal contact The graph shows that stainless steels have occurs frequently but is often not two ranges of potential. The usual, passive Galvanic corrosion can only occur if the a problem. The aluminium head behaviour is shown by the light hatching. dissimilar metals are in electrical contact. However, if the passive film breaks down, on a cast iron block, the solder on The contact may be direct or by an the stainless steel corrodes and its a copper pipe, galvanising on a external pipe or wire or bolt. If the potential is in the dark bar range. steel purlin and the steel fastener dissimilar metals are insulated from each in an aluminium sheet are common other by suitable plastic strips, washers or As a rule of thumb, if the potential examples. sleeves then galvanic corrosion cannot difference is less than 0.1 volt, then it is occur. Paint is not a reliable electrical unlikely that galvanic corrosion will be significant. WHAT CAUSES GALVANIC insulator especially under bolt heads or CORROSION? nuts or washers or near edges of sheets of If all three conditions are met then metal. The paint is usually damaged on galvanic corrosion is probable and the rate For galvanic or dissimilar or installation or by subsequent movement. of corrosion will be influenced by the electrolytic corrosion to occur, Note that the chromium oxide film layer relative area and the current density three conditions must be met: on the stainless steel is very thin and not delivered by the noble metal. -

Scholarship Essay Andreas Nilsson 3.31.14 Hot Dip Galvanization Is The
Scholarship Essay Andreas Nilsson 3.31.14 Hot dip galvanization is the process of dipping fabricated steel in molten zinc in order to protect the steel from corrosion. This process can be very beneficial to the architecture industry, and is critical that people understand the process in order be able to appreciate the benefits. Dipping the steel in zinc that has been raised to 830 degrees Fahrenheit allows the zinc to fully coat the steel and provide a barrier between the steel and the environmental elements that surround it. A common problem with steel is that it stands no chance when it comes into contact with moisture and oxygen. As the iron in the steel reacts with the oxygen and water, it produces a hydrated ferric oxide, also known as rust. Hot dip galvanization has been implemented for years in the industry to prevent this reaction of corrosion. Although the primary purpose of hot dip galvanization is to protect the steel from corrosion; there are also many other benefits such as providing a maintenance free and sustainable solution. The longevity of the materials used when erecting or designing a building is crucial. Since steel is made of iron, it reacts easily with moisture and air, which makes corrosion inevitable. Thankfully for the process of galvanizing metals; architects, contractors, and owners do not need to worry about the corrosion of their structures. Once the metal is fully coated in zinc, the metal will be protected from the surrounding environmental elements that threaten it. The zinc does not just offer protection from the environment by providing a barrier, but it also offers cathodic protection. -
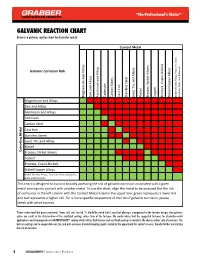
GALVANIC REACTION CHART Below Is a Galvanic Reaction Chart for Dissimilar Metals
GALVANIC REACTION CHART Below is a galvanic reaction chart for dissimilar metals. Contact Metal Silver, Silver, Galvanic Corrosion Risk Alloys Alloys Platinum Alloys Alloys, Titanium, and Alloys Steels Gold, and Iron Cast Stainless Steel Lead, Tin, and Magnesium and Zinc and Cadmium Carbon Nickel Brasses, Nickel-Silvers Copper Bronzes, Cupro-Nickels Nickel Copper Alloys Aluminum Graphite, Nickel-Chrome Magnesium and Alloys Zinc and Alloys Aluminum and Alloys Cadmium Carbon Steel Cast Iron Metal Stainless Steels Lead, Tin, and Alloys Nickel Corroding Brasses, Nickel-Silvers Copper Bronzes, Cupro-Nickels Nickel Copper Alloys Nickel-Chrome Alloys, Titanium, Silver, Graphite, Gold, and Platinum This chart is designed to assist in broadly assessing the risk of galvanic corrosion associated with a given metal coming into contact with another metal. To use the chart, align the metal to be assessed (for the risk of corrosion) in the left column with the Contact Metal listed in the upper row; green represents a lower risk and red represents a higher risk. For a more specific assessment of the risk of galvanic corrosion, please check with other sources. Please understand that green represents "lower risk" not "no risk." It should be noted that if sacrificial plating is incorporated in the fastener design, then galvanic action can result in the deterioration of the sacrificial coating, rather than of the fastener. We would advise that the suggested fasteners for dissimilar-metal applications would incorporate our GRABBERGARD® coating which utilizes both barrier and sacrificial coatings to minimize the chance and/or rate of corrosion. The barrier coating used to encapsulate our zinc and anti-corrosion chemical bonding agents minimize the opportunity for contact to occur, thereby further minimizing the risk of corrosion. -

An 18-Month Analysis of Bond Strength of Hot-Dip Galvanized Reinforcing Steel B500SP and S235JR+AR to Chloride Contaminated Concrete
materials Article An 18-Month Analysis of Bond Strength of Hot-Dip Galvanized Reinforcing Steel B500SP and S235JR+AR to Chloride Contaminated Concrete Mariusz Ja´sniok* , Jacek Kołodziej and Krzysztof Gromysz Faculty of Civil Engineering, Silesian University of Technology, 5 Akademicka, 44-100 Gliwice, Poland; [email protected] (J.K.); [email protected] (K.G.) * Correspondence: [email protected] Abstract: This article describes the comparative analysis of tests on bond strength of hot-dip galva- nized and black steel to concrete with and without chlorides. The bond effect was evaluated with six research methods: strength, electrochemical (measurements of potential, EIS and LPR), optical, and 3D scanning. The tests were conducted within a long period of 18 months on 48 test elements reinforced with smooth rebars φ8 mm from steel grade S235JR+AR and ribbed rebars φ8 mm and φ16 mm from steel grade B500SP. The main strength tests on the reinforcement bond to concrete were used to compare forces pulling out galvanized and black steel rebars from concrete. This comparative analysis was performed after 28, 180, and 540 days from the preparation of the elements. The electrochemical tests were performed to evaluate corrosion of steel rebars in concrete, particularly in chloride contaminated concrete. The behaviour of concrete elements while pulling out the rebar was observed using the system of digital cameras during the optical tests. As regards 3D scanning of ribbed rebars φ8 mm and φ16 mm, this method allowed the detailed identification of their complex geometry in terms of determining the polarization area to evaluate the corrosion rate of reinforcement Citation: Ja´sniok,M.; Kołodziej, J.; Gromysz, K. -

Development of a Low Resistance Low Corrosion Cathode Plate
Development of a low resistance, low corrosion cathode plate for electrowinning and electrorefining Nigel J. Aslin1*1, Christian Pasten2, Addin Pranowo3, Graham J. Heferen4 1. Glencore Technology, Australia ABSTRACT For nearly 40 years Glencore Technology (formerly Mount Isa Mines Copper Refineries) has been the mainstay supplier of permanent cathode plates to the copper industry with its ISA PROCESS and KIDD PROCESS cathode plates. Improvement to the cathode plate design remains a key area for research, and on-going developments by Glencore Technology have led to the commercialisation of a new design. The ISAKIDD cathode plate is universally suited to both electro-refining and electro- winning bringing major cost efficiencies to the operations through improvements in hanger bar strength, corrosion resistance and electrical conductivity. A variation of the this design uses a ‘steer- horn’ shaped hanger bar to lower the resistance path across the cathode plate giving significant tank- house power savings in electrowinning refineries. Technical aspects and results of commercial trials of the ISAKIDD and ‘HP’ cathode plate are discussed in this paper. A novel new contact system which allows shorting frames to be used with Stainless Steel hanger bars will also be tested. Trial results will demonstrate savings in a typical EW tankhouse of up to 720k USD per year in power costs alone, with further savings in maintenance costs and operational efficiencies. *Corresponding author: Glencore Technology, Technical Manager, c/- Copper Refineries Pty Ltd, Hunter St, Stuart, 4811, QLD, Australia. Phone: +61 418887034. Email: [email protected] 1 INTRODUCTION Glencore Technology were the pioneers of Permanent cathode technology for copper and remain committed to continuous improvement and innovation of the technology forty years later. -

Galvanic Corrosion
10 GALVANIC CORROSION X. G. ZHANG Teck Metals Ltd., Mississauga, Ontario, Canada A. Introduction graphite, are dispersed in a metal, or on a ship, where the B. Definition various components immersed in water are made of different C. Factors in galvanic corrosion metal alloys. In many cases, galvanic corrosion may result in D. Material factors quick deterioration of the metals but, in other cases, the D1. Effects of coupled materials galvanic corrosion of one metal may result in the corrosion D2. Effect of area protection of an attached metal, which is the basis of cathodic D3. Effect of surface condition protection by sacrificial anodes. E. Environmental factors Galvanic corrosion is an extensively investigated subject, E1. Effects of solution as shown in Table 10.1, and is qualitatively well understood E2. Atmospheric environments but, due to its highly complex nature, it has been difficult to E3. Natural waters deal with in a quantitative way until recently. The widespread F. Polarity reversal use of computers and the development of software have made G. Preventive measures great advances in understanding and predicting galvanic H. Beneficial effects of galvanic corrosion corrosion. I. Fundamental considerations I1. Electrode potential and Kirchhoff’s law I2. Analysis B. DEFINITION I3. Polarization and resistance I4. Potential and current distributions When two dissimilar conducting materials in electrical con- References tact with each other are exposed to an electrolyte, a current, called the galvanic current, flows from one to the other. Galvanic corrosion is that part of the corrosion that occurs at the anodic member of such a couple and is directly related to the galvanic current by Faraday’s law. -

Galvanic Corrosion Final
GALVANIC CORROSION by Stephen C. Dexter, Professor of Applied Science and Marine Biology, (302) 645-4261 Galvanic corrosion, often misnamed “electrolysis,” is one common form of corrosion in marine environments. It occurs Table 1 when two (or more) dissimilar metals are brought into electri- GALVANIC SERIES cal contact under water. When a galvanic couple forms, one of In Flowing Seawater the metals in the couple becomes the anode and corrodes faster than it would all by itself, while the other becomes the cathode Voltage Range of Alloy and corrodes slower than it would alone. Either (or both) metal Alloy vs. Reference Electrode* in the couple may or may not corrode by itself (themselves) in MagnesiumAnodic or -1.60 to -1.63 seawater. When contact with a dissimilar metal is made, how- Active End ever, the self-corrosion rates will change: corrosion of the Zinc -0.98 to -1.03 anode will accelerate; corrosion of the cathode will decelerate Aluminum Alloys -0.70 to -0.90 or even stop. We can use the seawater Galvanic Series, shown Cadmium -0.70 to -0.76 in Table 1, to predict which metal will become the anode and Cast Irons -0.60 to -0.72 how rapidly it will corrode. Steel -0.60 to -0.70 Aluminum Bronze -0.30 to -0.40 The seawater Galvanic Series is a list of metals and alloys Red Brass, Yellow Brass, ranked in order of their tendency to corrode in marine environ- Naval Brass -0.30 to -0.40 ments. If any two metals from the list are coupled together, the Copper -0.28 to -0.36 one closer to the anodic (or active) end of the series, the Lead-Tin Solder (50/50) -0.26 to -0.35 upper end in this case, will be the anode and thus will corrode Admiralty Brass -0.25 to -0.34 faster, while the one toward the cathodic (or noble) end will Manganese Bronze -0.25 to -0.33 corrode slower. -

Galvanic Corrosion Between Zinc and Carbon Steel Investigated by Local
Galvanic corrosion between zinc and carbon steel investigated by local electrochemical impedance spectroscopy Maixent Mouanga, Monique Puiggali, Bernard Tribollet, Vincent Vivier, Nadine Pébère, Olivier Devos To cite this version: Maixent Mouanga, Monique Puiggali, Bernard Tribollet, Vincent Vivier, Nadine Pébère, et al.. Gal- vanic corrosion between zinc and carbon steel investigated by local electrochemical impedance spec- troscopy. Electrochimica Acta, Elsevier, 2013, 88, pp.6-14. 10.1016/j.electacta.2012.10.002. hal- 01165531 HAL Id: hal-01165531 https://hal.archives-ouvertes.fr/hal-01165531 Submitted on 19 Jun 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Open Archive TOULOUSE Archive Ouverte ( OATAO ) OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible. This is an author-deposited version published in : http://oatao.univ-toulouse.fr/ Eprints ID : 14080 To link to this article : doi: 10.1016/j.electacta.2012.10.002 URL : http://dx.doi.org/10.1016/j.electacta.2012.10.002 To cite this version : Mouanga, Maixent and Puiggali, Monique and Tribollet, Bernard and Vivier, Vincent and Pébère, Nadine and Devos, Olivier Galvanic corrosion between zinc and carbon steel investigated by local electrochemical impedance spectroscopy . -

The Care of Historic Musical Instruments
The Care of Historic Musical Instruments Edited by Robert L. Barclay This publicatio11 lias bee11 produced by tile Museums & Galleries Commissio 11, the Ca11adia11 Co 11servatio11 IHstitute a11dthe IHtemati01 ml Commillee of Mu sical lllslmmelll Mu seums a11d Collectio11 s of the llltematiollal Cou 11 cil of Museums with fillallcial assista11ce from tile folm S. Colle11 Fou11da tio11. c .. -.HI I A ' b. u~t u t MUSEUMS & GALLERI ES Co..:u •\·.. uo~ CO:.IliiiSSION '''"".. " CIMCIM Edinburgh 1997 ©Her Majesty the Queen in Right of Canada, 1997, as represented by the Minister of the Department of Canadian Heritage acting through the Canadian Conservation Institute. Table of Contents ©Museums & Galleries Commission. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, by photocopying, recording or otherwise, for purposes of resale, without the prior Preface written permission of the copyright holders. All requests for permission must be directed to one of the following addresses: 1. Ethics and the Use of Instruments 1 Codes of Ethics and Standards 2 North America: All other countries and territories: Guidelines 3 Canadian Conservation Institute Museums & Galleries Commission Playability and "Soundability" 6 1030 Innes Road 16 Queen Anne's Gate Conclusion 7 Ottawa, Ontario KIA OMS London SW IH 9AA CANADA UNITED KINGDOM 2. Instruments in Their Environment 9 Conservation Assessment 9 Available for purchase from: Strategies for Environmental Control 11 Extension Services, Canadian Conservation Institute 3. General Care of Musical Instrument Collections 19 .Support for Display and Storage 19 Storage 22 Handling 23 Travel 24 Cataloguing in Publication Data Strategies to Counter Biological Attack 25 Main entry under title : 4. -

Development of Bath Chemical Composition for Batch Hot-Dip Galvanizing—A Review
materials Review Development of Bath Chemical Composition for Batch Hot-Dip Galvanizing—A Review Henryk Kania 1 , Jacek Mendala 2, Jarosław Kozuba 2 and Mariola Saternus 3,* 1 Department of Advanced Materials and Technology, Faculty of Engineering Materials, Silesian University of Technology, Krasi´nskiego8, 40-019 Katowice, Poland; [email protected] 2 Department of Aviation Technologies, Faculty of Transport and Aviation Engineering, Silesian University of Technology, Krasi´nskiego8, 40-019 Katowice, Poland; [email protected] (J.M.); [email protected] (J.K.) 3 Department of Metallurgy and Recycling, Faculty of Engineering Materials, Silesian University of Technology, Krasi´nskiego8, 40-019 Katowice, Poland * Correspondence: [email protected]; Tel.: +48-32-603-4275 Received: 26 August 2020; Accepted: 14 September 2020; Published: 19 September 2020 Abstract: Obtaining zinc coatings by the batch hot-dip galvanizing process currently represents one of the most effective and economical methods of protecting steel products and structures against corrosion. The batch hot-dip galvanizing process has been used for over 150 years, but for several decades, there has been a dynamic development of this technology, the purpose of which is to improve the efficiency of zinc use and reduce its consumption and improve the quality of the coating. The appropriate selection of the chemical composition of the galvanizing bath enables us to control the reactivity of steel, improve the drainage of liquid zinc from the product surface, and reduce the amount of waste, which directly affects the quality of the coating and the technology of the galvanizing process. For this purpose, the effect of many alloying additives to the zinc bath on the structure and thickness of the coating was tested. -

Galvanic Corrosion Dealloying Corrosion Velocity Phenomena
Galvanic Corrosion Dealloying Corrosion Velocity Phenomena Note: While this course does not contain proprietary BWRVIP or MRP information, per se, it does contain open literature information that was used in the creation of BWRVIP or MRP documents or BWRVIP or MRP information that was subsequently made non-proprietary via publication, etc. Corrosion and Corrosion Control in LWRs © 2011 by SIA, Inc. All rights reserved. Galvanic, Dealloying and Fluid Velocity Corrosion Learning Objectives • Understand the mechanism of galvanic corrosion ♦ Does “galvanic corrosion” always involve different metals? ♦ Is corrosion essentiallyyyg always galvanic corrosion? ♦ Cathodic protection • Identify dealloying corrosion • Understand the mechanism of the effects of fluid flow on corrosion ♦ Flow-accelerated corrosion (FAC) Corrosion and Corrosion Control in LWRs PRS-11-037 D BMG/ 2 © 2011 by SIA, Inc.. All rights reserved. Specific Forms of Corrosion 1. General or uniform corrosion 2. Galvanic corrosion 3. De-alloying corrosion Macro 4. Velocity phenomena - erosion corrosion, Localized Corrosion cavitation, impingement, fretting and FAC 5. Crevice corrosion 6. Pitting corrosion 7. Intergranular corrosion Micro Localized 8. Corrosion fatigue Corrosion 9. Stress corrosion cracking Microbiological activity can affect all of the above Corrosion and Corrosion Control in LWRs PRS-11-037 D BMG/ 3 © 2011 by SIA, Inc.. All rights reserved. Galvanic Corrosion Corrosion and Corrosion Control in LWRs © 2011 by SIA, Inc. All rights reserved. Galvanic Corrosion • History • Mechanism • LWR Case Study Examples ♦ Condensers ♦ Sensitization of stainless steel Corrosion and Corrosion Control in LWRs PRS-11-037 D BMG/ 5 © 2011 by SIA, Inc.. All rights reserved. Galvanic Corrosion • If you could fabricate a component from only one alloy, galvanic corrosion would not be a problem.