ECOLOGICAL IMPACT of MYRTLE RUST (Austropuccinia Psidii) in a WET SCLEROPHYLL FOREST
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TML Propagation Protocols
PROPAGATION PROTOCOLS This document is intended as a guide for Tamborine Mountain Landcare members who wish to assist our regeneration projects by growing some of the plants needed. It is a work in progress so if you have anything to add to the protocols – for example a different but successful way of propagating and growing a particular plant – then please give it to Julie Lake so she can add it to the document. The idea is that our shared knowledge and experience can become a valuable part of TML's intellectual property as well as a useful source of knowledge for members. As there are many hundreds of plants native to Tamborine Mountain, the protocols list will take a long time to complete, with growing information for each plant added alphabetically as time permits. While the list is being compiled by those members with competence in this field, any TML member with a query about propagating a particular plant can post it on the website for other me mb e r s to answer. To date, only protocols for trees and shrubs have been compiled. Vines and ferns will be added later. Fruiting times given are usual for the species but many rainforest plants flower and fruit opportunistically, according to weather and other conditions unknown to us, thus fruit can be produced at any time of year. Finally, if anyone would like a copy of the protocols, contact Julie on [email protected] and she’ll send you one. ………………….. Growing from seed This is the best method for most plants destined for regeneration projects for it is usually fast, easy and ensures genetic diversity in the regenerated landscape. -

Five Hundred Plant Species in Gunung Halimun Salak National Park, West Java a Checklist Including Sundanese Names, Distribution and Use
Five hundred plant species in Gunung Halimun Salak National Park, West Java A checklist including Sundanese names, distribution and use Hari Priyadi Gen Takao Irma Rahmawati Bambang Supriyanto Wim Ikbal Nursal Ismail Rahman Five hundred plant species in Gunung Halimun Salak National Park, West Java A checklist including Sundanese names, distribution and use Hari Priyadi Gen Takao Irma Rahmawati Bambang Supriyanto Wim Ikbal Nursal Ismail Rahman © 2010 Center for International Forestry Research. All rights reserved. Printed in Indonesia ISBN: 978-602-8693-22-6 Priyadi, H., Takao, G., Rahmawati, I., Supriyanto, B., Ikbal Nursal, W. and Rahman, I. 2010 Five hundred plant species in Gunung Halimun Salak National Park, West Java: a checklist including Sundanese names, distribution and use. CIFOR, Bogor, Indonesia. Photo credit: Hari Priyadi Layout: Rahadian Danil CIFOR Jl. CIFOR, Situ Gede Bogor Barat 16115 Indonesia T +62 (251) 8622-622 F +62 (251) 8622-100 E [email protected] www.cifor.cgiar.org Center for International Forestry Research (CIFOR) CIFOR advances human wellbeing, environmental conservation and equity by conducting research to inform policies and practices that affect forests in developing countries. CIFOR is one of 15 centres within the Consultative Group on International Agricultural Research (CGIAR). CIFOR’s headquarters are in Bogor, Indonesia. It also has offices in Asia, Africa and South America. | iii Contents Author biographies iv Background v How to use this guide vii Species checklist 1 Index of Sundanese names 159 Index of Latin names 166 References 179 iv | Author biographies Hari Priyadi is a research officer at CIFOR and a doctoral candidate funded by the Fonaso Erasmus Mundus programme of the European Union at Southern Swedish Forest Research Centre, Swedish University of Agricultural Sciences. -

MEDIA FACTSHEET Habitat Enhancement Efforts at Pulau Ubin
MEDIA FACTSHEET Habitat enhancement efforts at Pulau Ubin Reforestation efforts at Tanjong Tajam, Pulau Ubin 100 native trees were planted at Tanjong Tajam, Pulau Ubin as part of habitat enhancement efforts on 23 August 2014. Minister of State for National Development, Desmond Lee, planted a Pteleocarpa lamponga. About the Pteleocarpa lamponga The Pteleocarpa lamponga is a medium- sized tree with a bushy, open crown that can grow to more than 30m tall. Its leaves have a leathery texture. Bright yellow flowers are found in clusters at the ends of leafy twigs. Native to Singapore, and also found in Thailand, Malaysia, Sumatra and Borneo, the Pteleocarpa lamponga is presumed to be extinct in the wild in Singapore. Page 1 of 9 Aphanamixis polystachya (Common names: Pasak Lingga, Amoora, Cikih, Kasai Paya, Kulim Burung) Growing up to 20m tall, the leaves of the Aphanamixis polystachya are oblong and leathery. Its sweetly scented flowers are cream, yellow or bronze in colour. Oil extracted from its seeds have medicinal properties and used externally for rheumatism. However, its fruits are poisonous. The Aphanamixis polystachya is classified as endangered in Singapore. Erythroxylum cuneatum (Common names: Wild Cocaine, Baka, Beluntas Bukit, Cinatamula, Inai Inai, Mahang Wangi, Medang Lenggundi, Medang Wangi) Page 2 of 9 Growing up to 45m tall, the Erythroxylum cuneatum Has flattened green twigs. Its tiny flowers grow in clusters of 1-8, and are white to light green in colour. Its fruits are bright red when ripe, and are eaten by mammals and porcupines. The Erythroxylum cuneatum is classified as common in Singapore and can be found in Changi, Pulau Tekong, Pulau Ubin and St John’s Island. -

In China: Phylogeny, Host Range, and Pathogenicity
Persoonia 45, 2020: 101–131 ISSN (Online) 1878-9080 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE https://doi.org/10.3767/persoonia.2020.45.04 Cryphonectriaceae on Myrtales in China: phylogeny, host range, and pathogenicity W. Wang1,2, G.Q. Li1, Q.L. Liu1, S.F. Chen1,2 Key words Abstract Plantation-grown Eucalyptus (Myrtaceae) and other trees residing in the Myrtales have been widely planted in southern China. These fungal pathogens include species of Cryphonectriaceae that are well-known to cause stem Eucalyptus and branch canker disease on Myrtales trees. During recent disease surveys in southern China, sporocarps with fungal pathogen typical characteristics of Cryphonectriaceae were observed on the surfaces of cankers on the stems and branches host jump of Myrtales trees. In this study, a total of 164 Cryphonectriaceae isolates were identified based on comparisons of Myrtaceae DNA sequences of the partial conserved nuclear large subunit (LSU) ribosomal DNA, internal transcribed spacer new taxa (ITS) regions including the 5.8S gene of the ribosomal DNA operon, two regions of the β-tubulin (tub2/tub1) gene, plantation forestry and the translation elongation factor 1-alpha (tef1) gene region, as well as their morphological characteristics. The results showed that eight species reside in four genera of Cryphonectriaceae occurring on the genera Eucalyptus, Melastoma (Melastomataceae), Psidium (Myrtaceae), Syzygium (Myrtaceae), and Terminalia (Combretaceae) in Myrtales. These fungal species include Chrysoporthe deuterocubensis, Celoporthe syzygii, Cel. eucalypti, Cel. guang dongensis, Cel. cerciana, a new genus and two new species, as well as one new species of Aurifilum. These new taxa are hereby described as Parvosmorbus gen. -

Report on Biodiversity and Tropical Forests in Indonesia
Report on Biodiversity and Tropical Forests in Indonesia Submitted in accordance with Foreign Assistance Act Sections 118/119 February 20, 2004 Prepared for USAID/Indonesia Jl. Medan Merdeka Selatan No. 3-5 Jakarta 10110 Indonesia Prepared by Steve Rhee, M.E.Sc. Darrell Kitchener, Ph.D. Tim Brown, Ph.D. Reed Merrill, M.Sc. Russ Dilts, Ph.D. Stacey Tighe, Ph.D. Table of Contents Table of Contents............................................................................................................................. i List of Tables .................................................................................................................................. v List of Figures............................................................................................................................... vii Acronyms....................................................................................................................................... ix Executive Summary.................................................................................................................... xvii 1. Introduction............................................................................................................................1- 1 2. Legislative and Institutional Structure Affecting Biological Resources...............................2 - 1 2.1 Government of Indonesia................................................................................................2 - 2 2.1.1 Legislative Basis for Protection and Management of Biodiversity and -
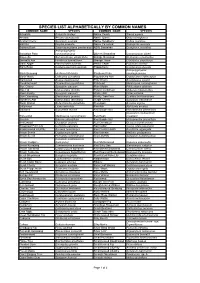
Species List Alphabetically by Common Names
SPECIES LIST ALPHABETICALLY BY COMMON NAMES COMMON NAME SPECIES COMMON NAME SPECIES Actephila Actephila lindleyi Native Peach Trema aspera Ancana Ancana stenopetala Native Quince Guioa semiglauca Austral Cherry Syzygium australe Native Raspberry Rubus rosifolius Ball Nut Floydia praealta Native Tamarind Diploglottis australis Banana Bush Tabernaemontana pandacaqui NSW Sassafras Doryphora sassafras Archontophoenix Bangalow Palm cunninghamiana Oliver's Sassafras Cinnamomum oliveri Bauerella Sarcomelicope simplicifolia Orange Boxwood Denhamia celastroides Bennetts Ash Flindersia bennettiana Orange Thorn Citriobatus pauciflorus Black Apple Planchonella australis Pencil Cedar Polyscias murrayi Black Bean Castanospermum australe Pepperberry Cryptocarya obovata Archontophoenix Black Booyong Heritiera trifoliolata Picabeen Palm cunninghamiana Black Wattle Callicoma serratifolia Pigeonberry Ash Cryptocarya erythroxylon Blackwood Acacia melanoxylon Pink Cherry Austrobuxus swainii Bleeding Heart Omalanthus populifolius Pinkheart Medicosma cunninghamii Blue Cherry Syzygium oleosum Plum Myrtle Pilidiostigma glabrum Blue Fig Elaeocarpus grandis Poison Corkwood Duboisia myoporoides Blue Lillypilly Syzygium oleosum Prickly Ash Orites excelsa Blue Quandong Elaeocarpus grandis Prickly Tree Fern Cyathea leichhardtiana Blueberry Ash Elaeocarpus reticulatus Purple Cherry Syzygium crebrinerve Blush Walnut Beilschmiedia obtusifolia Red Apple Acmena ingens Bollywood Litsea reticulata Red Ash Alphitonia excelsa Bolwarra Eupomatia laurina Red Bauple Nut Hicksbeachia -

Table of Contents Below) with Family Name Provided
1 Australian Plants Society Plant Table Profiles – Sutherland Group (updated August 2021) Below is a progressive list of all cultivated plants from members’ gardens and Joseph Banks Native Plants Reserve that have made an appearance on the Plant Table at Sutherland Group meetings. Links to websites are provided for the plants so that further research can be done. Plants are grouped in the categories of: Trees and large shrubs (woody plants generally taller than 4 m) Medium to small shrubs (woody plants from 0.1 to 4 m) Ground covers or ground-dwelling (Grasses, orchids, herbaceous and soft-wooded plants, ferns etc), as well as epiphytes (eg: Platycerium) Vines and scramblers Plants are in alphabetical order by botanic names within plants categories (see table of contents below) with family name provided. Common names are included where there is a known common name for the plant: Table of Contents Trees and Large shrubs........................................................................................................................... 2 Medium to small shrubs ...................................................................................................................... 23 Groundcovers and other ground‐dwelling plants as well as epiphytes. ............................................ 64 Vines and Scramblers ........................................................................................................................... 86 Sutherland Group http://sutherland.austplants.com.au 2 Trees and Large shrubs Acacia decurrens -

9 Costion Plant Endemism 133-166 PROOFS
Micronesica 41(1): 131–164, 2009 Plant Endemism, Rarity, and Threat in Palau, Micronesia: A Geographical Checklist and Preliminary Red List Assessment 1 CRAIG M. COSTION Department of Ecology and Evolutionary Biology, School of Earth and Environmental Sciences, University of Adelaide, Adelaide SA 5001 [email protected] ANN HILLMANN KITALONG The Environment, Inc., P.O. Box 1696, Koror, Palau 96940 TARITA HOLM Palau Conservation Society/PALARIS, P.O. Box 1811, Koror, Palau, 96940 Abstract—An official checklist of the endemic plant species of Palau has been long awaited, and is presented here for the first time. For each species a substrate limitation, growth form, and relative abundance is listed. In addition an IUCN red list assessment was conducted using all available data. For over half of the endemic species there is insufficient data to provide a red listing status however an expected minimum number of threatened plants out of the total is inferred. Approximately 15% of Palau’s endemic plants are believed to be only known from the type collection and many more only known from a few collections. These taxa however may now be prioritized and targeted for future inventory and research. The taxonomic robustness of several of these taxa is questionable and it is expected that more endemic species will be lost to synonymy in the future. Previous estimations have significantly over-estimated the rate of plant endemism in Palau (e.g., 194). Here, 130 plants are recognized for Palau, making its level of plant endem- ism comparable to some of its neighboring Micronesian islands to the east, notably Guam and Pohnpei. -

Species Selection Guidelines Tree Species Selection
Species selection guidelines Tree species selection This section of the plan provides guidance around the selection of species for use as street trees in the Sunshine Coast Council area and includes region-wide street tree palettes for specific functions and settings. More specific guidance on signature and natural character palettes and lists of trees suitable for use in residential streets for each of the region's 27 Local plan areas are contained within Part B – Street tree strategies of the plan. Street tree palettes will be periodically reviewed as an outcome of street tree trials, the development of new species varieties and cultivars, or the advent of new pest or disease threats that may alter the performance and reliability of currently listed species. The plan is to be used in association with the Sunshine Coast Council Open Space Landscape Infrastructure Manual where guidance for tree stock selection (in line with AS 2303–2018 Tree stock for landscape use) and tree planting and maintenance specifications can be found. For standard advanced tree planting detail, maintenance specifications and guidelines for the selection of tree stock see also the Sunshine Coast Open Space Landscape Infrastructure Manual – Embellishments – Planting Landscape). The manual's Plant Index contains a comprehensive list of all plant species deemed suitable for cultivation in Sunshine Coast amenity landscapes. For specific species information including expected dimensions and preferred growing conditions see Palettes – Planting – Planting index). 94 Sunshine Coast Street Tree Master Plan 2018 Part A Tree nomenclature Strategic outcomes The names of trees in this document follow the • Trees are selected by suitably qualified and International code of botanical nomenclature experienced practitioners (2012) with genus and species given, followed • Tree selection is locally responsive and by the plant's common name. -

Myrtle Rust Reviewed the Impacts of the Invasive Plant Pathogen Austropuccinia Psidii on the Australian Environment R
Myrtle Rust reviewed The impacts of the invasive plant pathogen Austropuccinia psidii on the Australian environment R. O. Makinson 2018 DRAFT CRCPLANTbiosecurity CRCPLANTbiosecurity © Plant Biosecurity Cooperative Research Centre, 2018 ‘Myrtle Rust reviewed: the impacts of the invasive pathogen Austropuccinia psidii on the Australian environment’ is licenced by the Plant Biosecurity Cooperative Research Centre for use under a Creative Commons Attribution 4.0 Australia licence. For licence conditions see: https://creativecommons.org/licenses/by/4.0/ This Review provides background for the public consultation document ‘Myrtle Rust in Australia – a draft Action Plan’ available at www.apbsf.org.au Author contact details R.O. Makinson1,2 [email protected] 1Bob Makinson Consulting ABN 67 656 298 911 2The Australian Network for Plant Conservation Inc. Cite this publication as: Makinson RO (2018) Myrtle Rust reviewed: the impacts of the invasive pathogen Austropuccinia psidii on the Australian environment. Plant Biosecurity Cooperative Research Centre, Canberra. Front cover: Top: Spotted Gum (Corymbia maculata) infected with Myrtle Rust in glasshouse screening program, Geoff Pegg. Bottom: Melaleuca quinquenervia infected with Myrtle Rust, north-east NSW, Peter Entwistle This project was jointly funded through the Plant Biosecurity Cooperative Research Centre and the Australian Government’s National Environmental Science Program. The Plant Biosecurity CRC is established and supported under the Australian Government Cooperative Research Centres Program. EXECUTIVE SUMMARY This review of the environmental impacts of Myrtle Rust in Australia is accompanied by an adjunct document, Myrtle Rust in Australia – a draft Action Plan. The Action Plan was developed in 2018 in consultation with experts, stakeholders and the public. The intent of the draft Action Plan is to provide a guiding framework for a specifically environmental dimension to Australia’s response to Myrtle Rust – that is, the conservation of native biodiversity at risk. -

Plants for Whitsunday Gardens
Whitsunday Plants [email protected] Suitable for Gardens Ph: (07) 4945 0267 What are Local Native Plants? Native plants are also far less Local native plants are those that are indigenous to the likely than exotic (non-native) Whitsunday region. There are many local native plants plants to become that can be used instead of exotic (introduced) plants or environmental weeds. Native even those native to other parts of Australia. Wedelia (pictured left) does not have the invasive qualities of the similar in appearance Why Use Local Native Plants? declared weed Singapore Local native plants are often better adapted to local Daisy. conditions and also provide a natural food source for Wedelia spilanthoides animals, birds and insects. It is important to plant a Photo: C. Peterson variety of plants to supply nectar, fruit and seeds to native animals. Things to Consider When Planning Below: Cajanus Your Garden: reticulatus, a low, Where space is limited such as in household gardens, dense shrub provides seeds to native under power lines and near driveways and buildings, animals. there is a need to use plants that will not become a Photo: P. Alden menace to neighbours or damage infrastructure. It is important to consider the mature size of plants, including width, height and how extensive the roots systems will become. Above: Melicope elleryana provides nectar, and later seeds to native Figs in particular (Ficus species) should not be planted animals. Photo: D. Pepplinkhouse near infrastructure. There are attractive grasses, ground covers, small to medium shrubs and small trees with colourful flowers, wonderful aromas or unusual fruit. -

Australian Nursery Industry Myrtle Rust Management Plan 2011
Australian Nursery Industry Myrtle Rust (Uredo rangelii) Management Plan 2011 Developed for the Australian Nursery Industry Production Wholesale Retail Acknowledgements This Myrtle Rust Management Plan has been developed by the Nursery & Garden Industry Queensland (John McDonald - Nursery Industry Development Manager) for the Australian Nursery Industry. Version 01 February 2011 Photographs sourced from I&I NSW and Queensland DEEDI. Various sources have contributed to the content of this plan including: • Nursery Industry Accreditation Scheme Australia (NIASA) • BioSecure HACCP • Nursery Industry Guava Rust Plant Pest Contingency Plan • DEEDI Queensland Myrtle Rust Fact Sheets • I&I NSW Myrtle Rust Fact Sheets and Updates Preparation of this document has been financially supported by Nursery & Garden Industry Queensland, Nursery & Garden Industry Australia and Horticulture Australia Ltd. Published by Nursery & Garden Industry Australia, Sydney 2011 © Nursery & Garden Industry Queensland 2011 While every effort has been made to ensure the accuracy of contents, Nursery & Garden Industry Queensland accepts no liability for the information. For further information contact: John McDonald Industry Development Manager NGIQ Ph: 07 3277 7900 Email: [email protected] 2 Nursery Industry Myrtle Rust Management Plan - 2011 Table of Contents 1. Managing Myrtle Rust in the Australian Nursery Industry 4 2. Myrtaceae Family – Genera 5 3. Myrtle Rust (Uredo rangelii) 6 4. Known Hosts of Myrtle Rust in Australia 7 5. Fungicide Treatment 8 5.2 Myrtle Rust Fungicide Treatment Rotation Program 8 5.3 Fungicide Application 9 6. On-site Biosecurity Actions 9 6.1 Production Nursery 10 6.2 Propagation (specifics) 11 6.3 Greenlife Markets/Retailers 12 7. Monitoring and Inspection Sampling Protocol 12 7.1 Monitoring Process 12 7.2 Sampling Process 13 8.