Inhalation Anesthetics & Vaporizers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Defeating Surgical Anguish: a Worldwide Tale of Creativity
Journal of Anesthesia and Patient Care Volume 3 | Issue 1 ISSN: 2456-5490 Research Article Open Access Defeating Surgical Anguish: A Worldwide Tale of Creativity, Hostility, and Discovery Iqbal Akhtar Khan*1 and Charles J Winters2 1Independent Scholar, Lahore, Pakistan 2Neurosurgeon, Washington County, 17-Western Maryland Parkway, Suit #100, Hagerstown, MD21740, United States *Corresponding author: Iqbal Akhtar Khan, MBBS, DTM, FACTM, PhD, Independent Scholar, Lahore, Pakistan, E-mail: [email protected] Citation: Iqbal Akhtar Khan, Charles J Winters (2018) Defeating Surgical Anguish: A Worldwide Tale of Creativity, Hostility, and Discovery. J Anesth Pati Care 3(1): 101 Received Date: March 01, 2018 Accepted Date: December 11, 2018 Published Date: December 13, 2018 In Memoria There are countless persons who have suffered through the ages around the world but not mentioned in any text or inscription. The following examples are sad but true tales of the journey through experimentation and torture. Ms. Eufame MacAlyane of Castle Hill Edinburg who, in 1591, was burned alive by order of the ruler of Scotland, King James I, who was an early opponent of “pain free labor”. Her “unforgivable offense” was to seek pain relief during labor [1]. Mrs. Kae Seishu volunteered as the brave first human subject to test “Tsusensan”, an oral anesthetic mixture formulated by her husband Dr. Seishu Hanaoka. The product met great success but she became permanently blind, presumably from repeated experimentation [2]. Their husbands’ agony and anguish is unimaginable! As such, it was a personalized, immeasurable, and unsharable experience. Apropos is a quote from an Urdu poet! Unknown remained their beloveds’ graves, Their nameless, traceless sanctuary. -

Alternative Methods to Teach History of Anesthesia
University of Massachusetts Medical School eScholarship@UMMS Anesthesiology and Perioperative Medicine Publications Anesthesiology and Perioperative Medicine 2012-10 Alternative Methods to Teach History of Anesthesia Manisha S. Desai University of Massachusetts Medical School Et al. Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/anesthesiology_pubs Part of the Anesthesiology Commons, and the Medical Education Commons Repository Citation Desai MS, Desai SP. (2012). Alternative Methods to Teach History of Anesthesia. Anesthesiology and Perioperative Medicine Publications. https://doi.org/10.13028/ng76-k913. Retrieved from https://escholarship.umassmed.edu/anesthesiology_pubs/142 This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in Anesthesiology and Perioperative Medicine Publications by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. AlternativeAlternative MethodsMethods toto TeachTeach HistoryHistory ofof AnesthesiaAnesthesia *Manisha S. Desai, MD and **Sukumar P. Desai, MD *Dept of Anesthesiology, UMass Memorial Health Care, University of Massachusetts Medical School, Worcester, MA; **Dept of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA Background: 3. Movies and video clips: Without disrupting the prime-time History of Anesthesia [HOA] may be taught through lectures, small schedule of grand rounds and other departmental lectures, we screen group discussions, or by one-on-one teaching. HOA competes for the 1944 Hollywood movie ‘The Great Moment,’3 starring James scarce time in a busy didactic schedule and for coverage in McCrea as William T. G. Morton, sometime in August for CA-1 mainstream medical journals devoted to anesthesiology. -

Basic Function of the Anesthetic Machine, Part II Some Vaporizers Are Filled with Liquid Agent by Using a Pin Fill Device
Vetamac Vapors (800)334-1583 www.vetamac.com Vol I, Issue 2 Welcome to the second quarterly edition of "Vetamac Vapors". The purpose of this newsletter is to educate your staff with a goal of the best possible patient care. We also would like to inform you of services and products that Vetamac has to offer and to provide support for all of your anesthesia needs. Future editions will cover topics on machines as well as different techniques. Please send comments to the e-mail link at www.vetamac.com. Basic Function of the Anesthetic Machine, Part II Some vaporizers are filled with liquid agent by using a pin fill device. There is an agent specific spout that replaces the cap on the The anesthetic machine must deliver vaporized anesthetic agent bottle. There is a keyed pin that fits into the fill manifold on the agent to the breathing circuit in concentrations that are optimal for the vaporizer. It is locked into place and the vaporizer is filled to the de- sired level on the window. Most vaporizers used in the U.S. have a desired effect. Since the liquid agent is in a closed system, a carrier funnel fill device and the liquid agent is simply poured into the vapor- gas must be present to deliver the vaporized agent to the breathing izer. Care must be exercised to avoid spillage. Anti-Spil™ adapters circuit. The gas used to accomplish this is the fresh gas flow of oxy- are available that replace the cap on the agent bottle and minimize the gen. -

Total Solutions for Anesthesia & Surgery
total solutions for Anesthesia & Surgery Ventilation Anesthesia Animal Surgery Temperature Control Physiological Monitoring NEW Catalogs Other Catalogs from Harvard Apparatus 2010 BTX Electroporation Catalog BTX offers a comprehensive line of instruments and accessories for both electroporation and electrofusion of mammalian, bacterial, yeast, fungi, insect and plant cells and tissues. BTX specializes in providing research tools for novel cutting edge applications such as adherent cell electroporation, high- throughput cloning, in vivo gene delivery, in ovo gene delivery, and in & ex utero gene delivery. 2009 Warner 2010 Animal, Organ & Cell Physiology Electrophysiology and This catalog features a broad range of products, including our Cell Biology Catalog legendary line of infusion and perfusion pumps, ventilators and anesthesia systems, surgical instruments and equipment for small For the electrophysiological, cellular, and to larger animals, and isolated organ and tissue systems for all neurological sciences. Our extensive levels of research and education. These products are designed to product line includes voltage and current help you achieve better research results in less time. clamp amplifiers for whole-cell and patch applications. Bessel filters, chambers for imaging and recording systems, perfusion control systems and steppers, 2010 Molecular solution heating systems, microscope translation tables, microelectrodes and holders, and glass capillary tubing, plus Sample Preparation now includes electroporation and transfection systems -
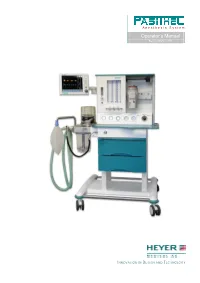
HEYER Pasithec
Anesthesia System Operator’s Manual Rev. 0.2 Draft – 12/09 INNOVATION IN DESIGN AND TECHNOLOGY HEYER Pasithec Contents 1 Statement .......................................................................................................................................................................... 5 1.1 Manufacturer Responsibility...................................................................................................................................... 5 1.2 Security, Reliability and Operating Conditions........................................................................................................... 5 1.3 Return ...................................................................................................................................................................... 6 1.4 Details of the Manufacturer....................................................................................................................................... 6 2 Introduction........................................................................................................................................................................ 7 2.1 Intended Use............................................................................................................................................................ 7 2.1.1 Range of Use .................................................................................................................................................. 7 2.1.2 Contraindication ............................................................................................................................................. -

CORRESPONDENCE Does Restrictive
Ⅵ CORRESPONDENCE Anesthesiology 2006; 104:889 © 2006 American Society of Anesthesiologists, Inc. Lippincott Williams & Wilkins, Inc. Does Restrictive Intraoperative Fluid Management Alter Outcome after Intraabdominal Surgery? To the Editor:—I read with great interest the recent article by Nisanev- confidence interval includes 1 and so would not be taken to indicate a ich et al.1 suggesting that restricted fluid therapy for intraabdominal statistical difference between the two therapies. surgery reduces postoperative morbidity. My comments focus on the The authors mention the use of the Newman-Keuls adjustment, but description of the statistical methods and their application to the data. that correction only applies if the group means are independent, The authors mention the use of both the chi-square test and Fisher which is clearly not the case here. Downloaded from http://pubs.asahq.org/anesthesiology/article-pdf/104/4/890/361043/0000542-200604000-00040.pdf by guest on 02 October 2021 exact test for the analysis of categorical data. However, it seems that No advanced statistical methods are used to model the data and only the results for the chi-square test are reported: For the number of explain the impact of relevant covariates. In particular, logistic regres- patients with complications, the Fisher exact test gives a nonsignificant sion could be used to model the presence of a complication on the P value of 0.056. The authors should explain why they report the number of fluid boluses, the degree of hypotension, the duration of results of one test and not the other. surgery, or American Society of Anesthesiologists physical status. -

ETHER in VOGUE: Nathan Cooley Keep and William Morton by Walter C
M ASSACHUSETTS GENERAL HOSPITAL BICENTENNIAL 1811-2011 “KEEP-ing” ETHER in VOGUE: Nathan Cooley Keep and William Morton By Walter C. Guralnick, DMD, and Leonard B. Kaban, DMD, MD This paper was presented originally at the 150th celebration of the first demonstration of ether anesthesia, Massachusetts General Hospital . For anyone connected with dentistry, celebrating the demonstration in 1846 by Boston dentist William Morton, is a memorable event. It is especially meaningful for those of us gathered here this evening in the historical Ether Dome. Particularly interesting in the Ether story is the role of Nathan Cooley Keep, an anesthesiologist and the first Dean of the Harvard Dental School. Furthermore, it will be enlightening to trace the estimable record of dentists and oral and maxillofacial surgeons in the administration of ambulatory anesthesia, a continuum of Morton’s watershed demonstration. Nathan Cooley Keep, who received an M.D. degree from the Harvard Medical School in 1827, was the leading dental practitioner of his era in Boston. He was born in Longmeadow, Massachusetts, a suburb of Springfield, in 1800. As a child, he was noted to have extraordinary mechanical skill. This ability was explained by The Historical and Genealogical Register (April 1878), in its memorial minute upon Dr. Keep’s death, as being inherited from his father who had “great ingenuity and mechanical skill.” Keep’s admirable humane qualities and his biological curiosity were also noted and ascribed to his mother, in the same document which stated: “… his own knowledge of disease; his fertility in suggesting relief in the sick room and his willingness and ability to lend personal help in relieving suffering in all forms, were a kind of natural inheritance from his mother.” Because of his skill with tools, young Keep was apprenticed at the age of 15 to a New Jersey jeweler. -

Interruption in the Supply of Breathing Gas During General Anesthesia Due to Malposition of the Vaporizer -A Case Report
Korean J Anesthesiol 2010 October 59(4): 270-274 Case Report DOI: 10.4097/kjae.2010.59.4.270 Interruption in the supply of breathing gas during general anesthesia due to malposition of the vaporizer -A case report- Hyo Jin Kim, and Mi Woon Kim Department of Anesthesiology and Pain Medicine, College of Medicine, Dongguk University, Gyeongju, Korea We report a case of interruption in the supply of breathing gas during general anesthesia caused by malposition of the Drager Vapor 2000Ⓡ vaporizer, which was accidentally tilted and lifted off the Selectatec manifold of the anesthesia machine. Because the patient was an 1-month-old infant, we couldn't check if he had experienced awareness with recall. We emphasize the importance of checking the anesthetic vaporizer after mounting it on the back bar of the anesthesia machine. (Korean J Anesthesiol 2010; 59: 270-274) Key Words: Gas leak, Selectatec, Vaporizer. The Selectatec, a vaporizer mounting system designed We experienced a case in which the Drager Vapor 2000Ⓡ by Datex-Ohmeda has several advantages over permanent vaporizer was incorrectly mounted on the Selectatec manifold mounting systems. It allows the vaporizer to be easily mounted, of the Datex-Ohmeda AestivaⓇ/5 compact plus anesthesia removed, and replaced, even during a case. The anesthesia machine, which caused a gas leak and interruption in the machine can have fewer mounting locations, allowing a more supply of breathing gas. compact machine. If malignant hyperthermia is suspected, the vaporizers can be removed. This gives better results than Case Report if the vaporizers remain on the machine in the off position [1]. -

Methods for Minimizing Pain Flare-Ups Survey
Methods for Minimizing Pain Flare‐Ups Survey I tried aqua therapy in a warm pool this past year. It helps to bypass my movement disorder. Not much minimizes my pain. I figure I'm going to be in pain doing nothing, and if I'm doing something. So I keep on keeping on. I decided to get a Harley Davidson a few years back and I ride to desensitize my nerves in my legs and helps to take my mind off it. Stay active regardless of the pain and reflect on what you accomplished instead of what you didn't. Even the smallest of things deserve your praise. Ketamine infusions are my monthly routine and I have them done now in the comfort of my own home. Water therapy is my biggest source of non‐medicinal pain relief. Moving around Warm weather, water therapy, and keeping busy to keep my mind off the pain. Also magnesium supplements to help me sleep better. Lavender lotion at night. Plenty of rest not just sleep but relax and rest throughout the day. It’s not just one thing that helps but a lot of little things. Don’t let the pain stop you although it may slow you down. Pain meds, antispasmodic pills, depression pills, anxiety pills, taking it easy not overdoing it, accepting it. Trusting God through each and every day. Nothing SO far, but I keep on keeping on the BEST I can I have found that two methods work best for me. I use a light therapy device that I actually bought from an infomercial when I was in desperate pain. -

DELISTING the DISHONORABLE Leah Deskins* INTRODUCTION
DELISTING THE DISHONORABLE Leah Deskins* INTRODUCTION ....................................................................................... 51 I. A BRIEF OVERVIEW OF THE CONFEDERATE MONUMENTS DEBATE .... 55 II. CONFEDERATE MONUMENTS CURRENTLY LISTED ON THE NATIONAL REGISTER........................................................................................... 60 III. SHOULD THE NATIONAL REGISTER LIST CONFEDERATE MONUMENTS? ........................................................................................................... 65 A. Confederate Monuments Do Not Meet the Requirements for National Register Listing ........................................................ 65 1. Confederate Monuments Fail to Qualify for Listing Pursuant to Criteria A and C ............................................................ 65 2. Confederate Monuments Do Not Satisfy Criteria Consideration F and They Need Not Remain Listed as Integral Parts of Listed Historic Districts ............................................... 69 B. American Society Has Rejected and Replaced References to the Confederacy ............................................................................ 71 C. But What of Heritage, History, and Memory?.............................. 75 IV. REMOVING CONFEDERATE MONUMENTS FROM THE NATIONAL REGISTER........................................................................................... 77 A. Delisting Is a Task for the National Park Service ........................ 78 B. Additional Information and Error in Professional Judgment -

SOP 1102 Surgivet® Anesthesia Machine
STANDARD OPERATING PROCEDURES DIVISION OF COMPARATIVE MEDICINE UNIVERSITY OF SOUTH FLORIDA SOP#: 1102.5 Date Issued: 5/99 Date Revised: 5/16 Page 1 of 3 TITLE: SurgiVet ™ Anesthesia Machine SCOPE: Research and Animal Care Personnel, RESPONSIBILITY: Facility Manager, Technical Staff, and Professional & Administrative Staff PURPOSE: To Outline the Proper Procedures for Use and Maintenance of Veterinary Inhalation Anesthesia Equipment I. PURPOSE 1. Isoflurane inhalation provides safe general anesthesia for a variety of animal species. This procedure outlines the use and maintenance of a veterinary inhalation anesthesia machine that incorporates an oxygen flowmeter, anesthetic vaporizer, and a circle re-breathing system with carbon dioxide absorption. II. RESPONSIBILITY 1. The Facility Manager ensures that equipment is appropriately cleaned, maintained in good working order, and available for research personnel as requested. 2. The veterinary professional, administrative, and managerial staff ensure that all research and technical staff using this equipment are adequately trained and experienced to perform veterinary inhalant general anesthesia. 3. The veterinary and technical staff operating this equipment ensure this procedure and the manufacturer’s operation manual are followed. III. EQUIPMENT SET- UP 1. Check that machine is connected to oxygen supply that is turned on and has an adequate supply at 50-55 psi. 2. Check the operation of the flow meter. 3. Check that vaporizer is adequately filled. 4. Check breathing circuit configuration and connections. 5. Check the scavenger system. Charcoal canisters should be weighed and replaced as recommended by the manufacturer. 6. Check ventilator function and connections. 7. Pressure test the machine as described in the operation manual. -

Fall 2005 Volume 6, Issue 2
The MASSACHUSETTS GENERAL HOSPITAL SURGICAL SOCIETY Newsletter Fall 2005 Volume 6, Issue 2 A MESSAGE FROM THE PRESIDENT -A GIFT OF THE SPIRIT Whether we are in practice, research, or education we all have the opportunity to leave legacies which will influence future generations. Our differing skills and interests will determine our con tributions. There are some surgeons who can transform an ordinary surgical operation into a work of art. There are others who have the ingenuity to develop needed new operations that will improve surgical care. Other surgeons have literary skills and can leave a valuable collection oftexts, ar ticles, mottoes and sayings that will be classic references for years to come. Still others make their contributions in research. Their fertile imaginative minds produce results that stimulate others to pursue their own unsolved quests. Although all of these legacies are important, in 1917 Moynihan pointed out that the greatest legacy that a surgeon can leave is "a gift of the spirit", the ability to inspire students with the highest ideals ofour profession. Ifthe surgeon shows kindness and concern for both his patients and his students he will develop a warmth and loyalty with them that will endure. The "gift of the spirit" is the greatest legacy you can leave. It will preserve surgery's soul. Reference: Moynihan, B, Obituary Theodor Kocher. Brit Med Journal, pages 168-169, 1917. Robb Rutledge. THIRD MEETING OF THE MGH SURGICAL SOCIETY JUNE 10-12, 2005 BOSTON, MASSACHUSETTS The meeting activities started with a welcome reception at the Wang Lobby on the evening of June 10,2005.