The Development of a Water Quality Model for Baltimore Harbor, Back River, and the Adjacent Upper Chesapeake Bay
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

No-Discharge Zones for Vessel Sewage in Maryland and Virginia
This document is scheduled to be published in the Federal Register on 05/11/2021 and available online at federalregister.gov/d/2021-09957, and on govinfo.gov 6560-50-P ENVIRONMENTAL PROTECTION AGENCY [FRL 10021-74-Region 3] Clean Water Act: No-Discharge Zones for Vessel Sewage in Maryland and Virginia AGENCY: Environmental Protection Agency (EPA). ACTION: Notice–final determination. SUMMARY: On behalf of the State of Maryland, the Secretary of the Maryland Department of Natural Resources requested that the Regional Administrator, U.S. Environmental Protection Agency, Region 3 approve a no-discharge zone for thirteen water bodies in Anne Arundel County, Maryland pursuant to the Clean Water Act. After review of Maryland’s application, EPA determined that adequate facilities for the safe and sanitary removal and treatment of sewage from all vessels are reasonable available for all thirteen waterbodies within Anne Arundel County. The application is available upon request from EPA (at the email address below). DATES: This approval is effective upon the date of publication in the Federal Register on [INSTERT DATE OF PUBLICATION IN THE FEDERAL REGISTER]. FOR FURTHER INFORMATION CONTACT: Ferry Akbar Buchanan, U. S. Environmental Protection Agency – Region III. Telephone: (215) 814-2570; email address: [email protected]. SUPPLEMENTARY INFORMATION: Pursuant to Clean Water Act section 312(f)(3), if any state determines that the protection and enhancement of the quality of some or all of the state’s waters require greater environmental protection, the state may designate the waters as a vessel sewage no-discharge zone. However, the state may not establish the no-discharge zone until EPA has determined that adequate pumpout facilities for the safe and sanitary removal and treatment of sewage from all vessels are reasonably available for the proposed waters. -

2012-AG-Environmental-Audit.Pdf
TABLE OF CONTENTS INTRODUCTION .............................................................................................................. 1 CHAPTER ONE: YOUGHIOGHENY RIVER AND DEEP CREEK LAKE .................. 4 I. Background .......................................................................................................... 4 II. Active Enforcement and Pending Matters ........................................................... 9 III. The Youghiogheny River/Deep Creek Lake Audit, May 16, 2012: What the Attorney General Learned............................................................................................. 12 CHAPTER TWO: COASTAL BAYS ............................................................................. 15 I. Background ........................................................................................................ 15 II. Active Enforcement Efforts and Pending Matters ............................................. 17 III. The Coastal Bays Audit, July 12, 2012: What the Attorney General Learned .. 20 CHAPTER THREE: WYE RIVER ................................................................................. 24 I. Background ........................................................................................................ 24 II. Active Enforcement and Pending Matters ......................................................... 26 III. The Wye River Audit, October 10, 2012: What the Attorney General Learned 27 CHAPTER FOUR: POTOMAC RIVER NORTH BRANCH AND SAVAGE RIVER 31 I. Background ....................................................................................................... -

Gunpowder River
Table of Contents 1. Polluted Runoff in Baltimore County 2. Map of Baltimore County – Percentage of Hard Surfaces 3. Baltimore County 2014 Polluted Runoff Projects 4. Fact Sheet – Baltimore County has a Problem 5. Sources of Pollution in Baltimore County – Back River 6. Sources of Pollution in Baltimore County – Gunpowder River 7. Sources of Pollution in Baltimore County – Middle River 8. Sources of Pollution in Baltimore County – Patapsco River 9. FAQs – Polluted Runoff and Fees POLLUTED RUNOFF IN BALTIMORE COUNTY Baltimore County contains the headwaters for many of the streams and tributaries feeding into the Patapsco River, one of the major rivers of the Chesapeake Bay. These tributaries include Bodkin Creek, Jones Falls, Gwynns Falls, Patapsco River Lower North Branch, Liberty Reservoir and South Branch Patapsco. Baltimore County is also home to the Gunpowder River, Middle River, and the Back River. Unfortunately, all of these streams and rivers are polluted by nitrogen, phosphorus and sediment and are considered “impaired” by the Maryland Department of the Environment, meaning the water quality is too low to support the water’s intended use. One major contributor to that pollution and impairment is polluted runoff. Polluted runoff contaminates our local rivers and streams and threatens local drinking water. Water running off of roofs, driveways, lawns and parking lots picks up trash, motor oil, grease, excess lawn fertilizers, pesticides, dog waste and other pollutants and washes them into the streams and rivers flowing through our communities. This pollution causes a multitude of problems, including toxic algae blooms, harmful bacteria, extensive dead zones, reduced dissolved oxygen, and unsightly trash clusters. -

Maryland Stream Waders 10 Year Report
MARYLAND STREAM WADERS TEN YEAR (2000-2009) REPORT October 2012 Maryland Stream Waders Ten Year (2000-2009) Report Prepared for: Maryland Department of Natural Resources Monitoring and Non-tidal Assessment Division 580 Taylor Avenue; C-2 Annapolis, Maryland 21401 1-877-620-8DNR (x8623) [email protected] Prepared by: Daniel Boward1 Sara Weglein1 Erik W. Leppo2 1 Maryland Department of Natural Resources Monitoring and Non-tidal Assessment Division 580 Taylor Avenue; C-2 Annapolis, Maryland 21401 2 Tetra Tech, Inc. Center for Ecological Studies 400 Red Brook Boulevard, Suite 200 Owings Mills, Maryland 21117 October 2012 This page intentionally blank. Foreword This document reports on the firstt en years (2000-2009) of sampling and results for the Maryland Stream Waders (MSW) statewide volunteer stream monitoring program managed by the Maryland Department of Natural Resources’ (DNR) Monitoring and Non-tidal Assessment Division (MANTA). Stream Waders data are intended to supplementt hose collected for the Maryland Biological Stream Survey (MBSS) by DNR and University of Maryland biologists. This report provides an overview oft he Program and summarizes results from the firstt en years of sampling. Acknowledgments We wish to acknowledge, first and foremost, the dedicated volunteers who collected data for this report (Appendix A): Thanks also to the following individuals for helping to make the Program a success. • The DNR Benthic Macroinvertebrate Lab staffof Neal Dziepak, Ellen Friedman, and Kerry Tebbs, for their countless hours in -

Analysis of the Jones Falls Sewershed
GREEN TOWSON ALLIANCE WHITE PAPER ON SEWERS AUGUST 31, 2017 IS RAW SEWAGE CONTAMINATING OUR NEIGHBORHOOD STREAMS? ANALYISIS OF THE JONES FALLS SEWERSHED ADEQUATE PUBLIC SEWER TASK FORCE OF THE GREEN TOWSON ALLIANCE: Larry Fogelson, Retired MD State Planner, Rogers Forge Resident Tom McCord, Former CFO, and MD Industry CPA, Ruxton Resident Roger Gookin, Retired Sewer Contractor, Towsongate Resident JONES FALLS SEWER SYSTEM INVESTIGATION AND REPORT It is shocking that in the year 2017, human waste is discharged into open waters. The Green Towson Alliance (GTA) believes the public has a right to know that continuing problems with the Baltimore County sanitary sewer system are probably contributing to dangerous bacteria in Lake Roland and the streams and waterways that flow into it. This paper is the result of 15 months of inquiries and analysis of information provided by the County and direct observations by individuals with expertise in various professional disciplines related to this topic. GTA welcomes additional information that might alter our analysis and disprove our findings. To date, the generalized types of responses GTA has received to questions generated by our analysis do not constitute proof, or provide assurance, that all is well. 1. The Adequate Public Sewer Task Force of the Green Towson Alliance: The purpose of this task force is to protect water quality and public health by promoting the integrity and adequacy of the sanitary sewer systems that serve the Towson area. It seeks transparency by regulators and responsible officials to assure that the sewerage systems are properly planned, maintained, and operated in a manner that meets the requirements of governing laws and regulations. -

Nesting in Chesapeake Bay Regions of Concern
W&M ScholarWorks VIMS Articles Virginia Institute of Marine Science 9-2015 Decadal re-evaluation of contaminant exposure and productivity of ospreys (Pandion haliaetus) nesting in Chesapeake Bay Regions of Concern RS Lazarus BA Rattner PC McGowan Robert Hale Virginia Institute of Marine Science et al Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Environmental Sciences Commons Recommended Citation Lazarus, RS; Rattner, BA; McGowan, PC; Hale, Robert; and al, et, "Decadal re-evaluation of contaminant exposure and productivity of ospreys (Pandion haliaetus) nesting in Chesapeake Bay Regions of Concern" (2015). VIMS Articles. 1661. https://scholarworks.wm.edu/vimsarticles/1661 This Article is brought to you for free and open access by the Virginia Institute of Marine Science at W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Environmental Pollution 205 (2015) 278e290 Contents lists available at ScienceDirect Environmental Pollution journal homepage: www.elsevier.com/locate/envpol Decadal re-evaluation of contaminant exposure and productivity of ospreys (Pandion haliaetus) nesting in Chesapeake Bay Regions of Concern * Rebecca S. Lazarus a, b, Barnett A. Rattner a, , Peter C. McGowan c, Robert C. Hale d, Sandra L. Schultz a, Natalie K. Karouna-Renier a, Mary Ann Ottinger b, 1 a U.S. Geological Survey, Patuxent Wildlife Research Center, Beltsville, MD 20705, USA b Marine-Estuarine Environmental Sciences Program and Department of Animal and Avian Sciences, University of Maryland, College Park, MD 20742, USA c U.S. Fish and Wildlife Service, Chesapeake Bay Field Office, Annapolis, MD 21401, USA d Virginia Institute of Marine Science, College of William and Mary, Gloucester Point, VA 23062, USA article info abstract Article history: The last large-scale ecotoxicological study of ospreys (Pandion haliaetus) in Chesapeake Bay was con- Received 30 March 2015 ducted in 2000e2001 and focused on U.S. -
Water Resources
2009 COMPREHENSIVE PLAN Water Resources 2009 CITY OF WESTMINSTER Water Resources 2009 What is the Water Resources Element? Community Vision for During its 2008 General Session, the Maryland General Water Assembly, as part of section 1.03 (iii) of Article 66B of the Annotated Code of Maryland, mandated that all Maryland The City’s ability to provide water to counties and municipalities exercising planning and residents and businesses has become an zoning authority prepare and adopt a Water Resources issue of great importance in recent years. Element in their Comprehensive Plans. According to the 2008 Community Survey, 65% of residents are satisfied with Requirements: the quality of water in Westminster, while Identify drinking water and other water resources others were concerned with the quality of that will be adequate for the needs of existing and their water service. future development Residents would like to improved water Identify suitable receiving waters and land areas to service in terms of improved meet the storm water management and wastewater communication. For example, residents treatment and disposal needs of existing and future development would like the City give proper notification when performing sewer or Purpose: water system repairs, as those repairs affect water pressure or the color of water. To ensure the Comprehensive Plan integrates water resources issues and potential solutions Residents also suggested that the City create incentives for residents to conserve To outline how management of water, wastewater and stormwater will support planned growth, given water in order to ensure reliable water water resource limitations service can continue into the future. Water Resources Priorities: State Planning Visions found in this Element: Water supply availability Infrastructure: Growth areas have the water resources and infrastructure to accommodate Reclaimed water use population and business expansion in an orderly, efficient, and environmentally sound manner. -

Sewage and Wastewater Plants in the Chesapeake Bay Watershed 21 Sewage Plants Violated Permit Limits in 2016; PA and VA Used Trading to Allow Pollution
Sewage and Wastewater Plants in the Chesapeake Bay Watershed 21 Sewage Plants Violated Permit Limits in 2016; PA and VA Used Trading to Allow Pollution NOVEMBER 29, 2017 ACKNOWLEDGEMENTS This report was researched and written by Courtney Bernhardt and Tom Pelton of the Environmental Integrity Project. THE ENVIRONMENTAL INTEGRITY PROJECT The Environmental Integrity Project (http://www.environmentalintegrity.org) is a nonpartisan, nonprofit organization established in March of 2002 by former EPA enforcement attorneys to advocate for effective enforcement of environmental laws. EIP has three goals: 1) to provide objective analyses of how the failure to enforce or implement environmental laws increases pollution and affects public health; 2) to hold federal and state agencies, as well as individual corporations, accountable for failing to enforce or comply with environmental laws; and 3) to help local communities obtain the protection of environmental laws. For questions about this report, please contact EIP Director of Communications Tom Pelton at (202) 888-2703 or [email protected]. PHOTO CREDITS Cover photo of Patapsco Wastewater Treatment Plant from University of Maryland Center for Environmental Science. Photo of Back River WWTP by Tom Pelton. Photo of Monocacy River by Maryland Department of Natural Resources. Photo of Massanutten Wastewater Treatment Plant by Alan Lehman. Sewage and Wastewater Plants in the Chesapeake Bay Watershed Executive Summary Across the Chesapeake Bay watershed, 21 sewage treatment plants violated their permit limits last year by releasing excessive amounts of nitrogen or phosphorus pollution that fuel algal blooms and low-oxygen “dead zones” in waterways, according to an Environmental Integrity Project examination of federal and state records.1 The plants in violation included 12 municipal sewage facilities in Maryland that treat more than half of the state’s wastewater, with the most pollution coming from the state’s largest two facilities: Baltimore’s Back River and Patapsco wastewater treatment plants. -
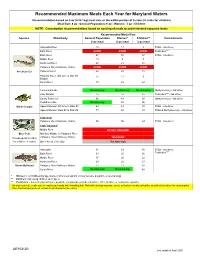
Recommended Maximum Fish Meals Each Year For
Recommended Maximum Meals Each Year for Maryland Waters Recommendation based on 8 oz (0.227 kg) meal size, or the edible portion of 9 crabs (4 crabs for children) Meal Size: 8 oz - General Population; 6 oz - Women; 3 oz - Children NOTE: Consumption recommendations based on spacing of meals to avoid elevated exposure levels Recommended Meals/Year Species Waterbody General PopulationWomen* Children** Contaminants 8 oz meal 6 oz meal 3 oz meal Anacostia River 15 11 8 PCBs - risk driver Back River AVOID AVOID AVOID Pesticides*** Bush River 47 35 27 PCBs - risk driver Middle River 13 9 7 Northeast River 27 21 16 Patapsco River/Baltimore Harbor AVOID AVOID AVOID American Eel Patuxent River 26 20 15 Potomac River (DC Line to MD 301 1511 9 Bridge) South River 37 28 22 Centennial Lake No Advisory No Advisory No Advisory Methylmercury - risk driver Lake Roland 12 12 12 Pesticides*** - risk driver Liberty Reservoir 96 48 48 Methylmercury - risk driver Tuckahoe Lake No Advisory 93 56 Black Crappie Upper Potomac: DC Line to Dam #3 64 49 38 PCBs - risk driver Upper Potomac: Dam #4 to Dam #5 77 58 45 PCBs & Methylmercury - risk driver Crab meat Patapsco River/Baltimore Harbor 96 96 24 PCBs - risk driver Crab "mustard" Middle River DO NOT CONSUME Blue Crab Mid Bay: Middle to Patapsco River (1 meal equals 9 crabs) Patapsco River/Baltimore Harbor "MUSTARD" (for children: 4 crabs ) Other Areas of the Bay Eat Sparingly Anacostia 51 39 30 PCBs - risk driver Back River 33 25 20 Pesticides*** Middle River 37 28 22 Northeast River 29 22 17 Brown Bullhead Patapsco River/Baltimore Harbor 17 13 10 South River No Advisory No Advisory 88 * Women = of childbearing age (women who are pregnant or may become pregnant, or are nursing) ** Children = all young children up to age 6 *** Pesticides = banned organochlorine pesticide compounds (include chlordane, DDT, dieldrin, or heptachlor epoxide) As a general rule, make sure to wash your hands after handling fish. -

Keep Maryland Beautiful Award Recipients
Protecting Land Forever Keep Maryland Beautiful Award Recipients Fiscal Year 2019 Bill James Environmental Grants Historic Sotterley, Inc Howard County Antique Farm Machinery Club Mountain Laurel Garden Club North County High School Pocomoke Middle School Clean Up & Green Up Maryland Grants African American Firefighters Historical Society Alice Ferguson Foundation Allegany County Commissioners & the Allegany County Solid Waste Management Board Annapolis Arts District Annapolis Green, Inc. Antietam-Conococheague Watershed Alliance Back River Restoration Committee, Inc. Banner Neighborhoods Bel Air Downtown Alliance Bethesda Green Beyond the Classroom, Inc. Brunswick Main Street, Inc. BUILD - Rebuild Johnston Square Neighborhood Org C.A.R.E Community Association, Inc Centreville Main Street Town of Centreville City of Greenbelt Department of Public Works Downtown Frederick Partnership Downtown Sykesville Connection at the Community Foundation of Carroll County Druid Heights Community Development Corporation Dundalk Renaissance Corporation Elkton Alliance, Inc. Fusion Partnerships, Inc. (Whitelock Community Farm) Galena Tree and Park Committee Havre de Grace Citizens Against Trash Historic Frostburg - a Maryland Main Street Community Howard County Conservancy I'm Still Standing By Grace Intersection of Change, Inc. Let's Beautify Cumberland! Main Street Historic Chestertown Main Street Middletown, MD Inc Main Street Princess Anne Metropolitan Washington Council of Governments Milton Montford Montgomery Parks Foundation Park Heights Renaissance Pigtown Main Street, Inc. Sandtown South Neighborhood Alliance Southeast Community Development Corporation Strong City Baltimore Takoma/Langley Crossroads Development Authority, Inc. The 6th Branch The Town of Colmar Manor Town of Emmitsburg Town of Manchester Town of Oakland Town of Thurmont & Main Street Westport Community Economic Development Corporation Margaret Rosch Jones Awards All Saints Episcopal Church Cool Green Schools Maryland Coastal Bays Program Sky Valley Association, Inc. -

Watersheds.Pdf
Watershed Code Watershed Name 02130705 Aberdeen Proving Ground 02140205 Anacostia River 02140502 Antietam Creek 02130102 Assawoman Bay 02130703 Atkisson Reservoir 02130101 Atlantic Ocean 02130604 Back Creek 02130901 Back River 02130903 Baltimore Harbor 02130207 Big Annemessex River 02130606 Big Elk Creek 02130803 Bird River 02130902 Bodkin Creek 02130602 Bohemia River 02140104 Breton Bay 02131108 Brighton Dam 02120205 Broad Creek 02130701 Bush River 02130704 Bynum Run 02140207 Cabin John Creek 05020204 Casselman River 02140305 Catoctin Creek 02130106 Chincoteague Bay 02130607 Christina River 02050301 Conewago Creek 02140504 Conococheague Creek 02120204 Conowingo Dam Susq R 02130507 Corsica River 05020203 Deep Creek Lake 02120202 Deer Creek 02130204 Dividing Creek 02140304 Double Pipe Creek 02130501 Eastern Bay 02141002 Evitts Creek 02140511 Fifteen Mile Creek 02130307 Fishing Bay 02130609 Furnace Bay 02141004 Georges Creek 02140107 Gilbert Swamp 02130801 Gunpowder River 02130905 Gwynns Falls 02130401 Honga River 02130103 Isle of Wight Bay 02130904 Jones Falls 02130511 Kent Island Bay 02130504 Kent Narrows 02120201 L Susquehanna River 02130506 Langford Creek 02130907 Liberty Reservoir 02140506 Licking Creek 02130402 Little Choptank 02140505 Little Conococheague 02130605 Little Elk Creek 02130804 Little Gunpowder Falls 02131105 Little Patuxent River 02140509 Little Tonoloway Creek 05020202 Little Youghiogheny R 02130805 Loch Raven Reservoir 02139998 Lower Chesapeake Bay 02130505 Lower Chester River 02130403 Lower Choptank 02130601 Lower -

The Iron Ores of Maryland, with an Account of the Iron Industry
Hass 77,>0 3- Book ffjZd6 MARYLAND GEOLOGICAL AND ECONOMIC SURVEY WM. BULLOCK CLARK, State Geologist REPORT ON THE IRON ORES OF MARYLAND WITH AN ACCOUNT OF THE IRON INDUSTRY BY JOSEPH T. SINGEWALD, JR.- (Special Publication, Volume IX, Part III) THE JOHNS HOPKINS PRESS Baltimore, December, 1911 / / MARYLAND GEOLOGICAL AND ECONOMIC SURVEY WM. BULLOCK CLARK, State Geologist REPORT ON r? Sr THE IRON ORES OF MARYLAND / £ WITH AN ACCOUNT OF THE IRON INDUSTRY BY JOSEPH T. SINGEWALD, JR. M (Special Publication, Volume IX, Part III) THE JOHNS HOPKINS PRESS • Baltimore, December, 1911 V n, ffi ft- sre so i CONTENTS PAGE PART III. REPORT ON THE IRON ORES OF MARYLAND, WITH AN ACCOUNT OF THE IRON INDUSTRY. By Joseph T. Sxngewald, Jr. 121 The Ores of Iron.123 Magnetite . 124 Hematite . 124 Limonite . 124 Carbonate or Siderite. 125 Impurities in the Ores and Their Effects. 125 Mechanical Impurities. 125 Chemical Impurities. 126 Practical Considerations. 127 History of the Maryland Iron Industry. 128 The Colonial Period. 128 The Period from 1780 to 1830. 133 • The Period from 1830 to 1885. 133 The Period from 1885 to the present time. 136 Description of Maryland Iron Works.139 Maryland Furnaces. 139 Garrett County..'... 139 Allegany County. 139 Washington County. 143 Frederick County. 146 Carroll County. 149 Baltimore County. 150 Baltimore City. 159 Harford County. 160 Cecil County. 162 Howard County. 168 Anne Arundel County. 169 Prince George’s County. 171 Worcester County. 172 9 Other Iron Works in Maryland. 173 Allegany County. 173 Baltimore County. 173 Cecil County. 174 CONTENTS PAGE Queen Anne’s County.