The Iron-Silver Processes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tucson Art Academy Online Skip Whitcomb
TUCSON ART ACADEMY ONLINE SKIP WHITCOMB PAINTS WHITE Any good to professional quality Titanium or Titanium/Zinc White in large tubes(150-200ML) size. Jack Richeson Co., Gamblin, Vasari, Utrecht, Winsor & Newton are all good brands, as are several other European manufacturers. I strongly recommend staying away from student grade paints, they do not mix or handle the same as higher/professional grade paints. YELLOWS Cadmium Yellow Lemon Cadmium Yellow Lt. (warm) Cad. Yellow Medium or Deep Indian Yellow ORANGES Cadmium Yellow Orange (optional) Cadmium Orange REDS Cadmium Red Light/ Pale/ Scarlet (warm) Cadmium Red Deep Permanent Alizarin Crimson Permanent Rose (Quinacridone) BLUES Ultramarine Blue Deep or Dark Cobalt Blue Prussian Blue or Phthalo Blue GREENS Viridian Viridian Hue (Phthalo Green) Chrome Oxide Green Olive Green Sap Green Yellow Green VIOLETS Mauve Blue Shade (Winsor&Newton) Dioxazine Violet or Purple EARTH COLORS Yellow Ochre Raw Sienna Raw Umber Burnt Sienna Terra Rosa Indian Red Venetian Red Burnt Umber Van Dyke Brown BLACKS Ivory Black Mars Black Chromatic Black Blue Black MARS COLORS Mars Yellow Mars Orange Mars Red Mars Violet IMPORTANT TO NOTE!! Please don’t be intimidated by this list! You will not be required to have all these colors on hand for our class. This is intended to be a recommendation for the studio. Specific colors on this list will come in handy for mixing in certain color plans. I will be happy to make suggestions along the way A good working palette for the studio would be: Cad. Yellow Lemon, Cad. Yellow Pale(warm), and/or Cad. -

The Art-Union and Photography, 1839-1854: the First Fifteen Years Of
THE ART-UNION AND PHOTOGRAPHY, 1839-1854: THE FIRST FIFTEEN YEARS OF CRITICAL ENGAGEMENT BETWEEN TWO CULTURAL ICONS OF NINETEENTH-CENTURY BRITAIN Derek Nicholas Boetcher, B.A., M.A. Thesis Prepared for the Degree of MASTER OF ARTS UNIVERSITY OF NORTH TEXAS August 2011 APPROVED: Denis Paz, Major Professor Denise Amy Baxter, Minor Professor Olga Velikanova, Committee Member Richard B. McCaslin, Chair of the Department of History James D. Meernik, Acting Dean of the Toulouse Graduate School Boetcher, Derek Nicholas. The Art-Union and Photography, 1839-1854: The First Fifteen Years of Critical Engagement between Two Cultural Icons of Nineteenth-Century Britain. Master of Arts (History), August 2011, 163 pp., bibliography, 69 titles. This study analyzes how the Art-Union, a British journal interested only in the fine arts, approached photography between 1839 and 1854. It is informed by Karl Marx’s materialism- informed commodity fetishism, Gerry Beegan’s conception of knowingness, Benedict Anderson’s imagined community, and an art critical discourse that was defined by Roger de Piles and Joshua Reynolds. The individual chapters are each sites in which to examine these multiple theoretical approaches to the journal’s and photography’s association in separate, yet sometimes overlapping, periods. One particular focus of this study concerns the method through which the journal viewed photography—as an artistic or scientific enterprise. A second important focus of this study is the commodification of both the journal and photography in Britain. Also, it determines how the journal’s critical engagement with photography fits into the structure and development of a nineteenth-century British social collectivity focused on art and the photographic enterprise. -
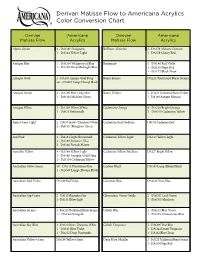
Derivan Matisse Flow to Americana Acrylics Color Conversion Chart
Derivan Matisse Flow to Americana Acrylics Color Conversion Chart Derivan Americana Derivan Americana Matisse Flow Acrylics Matisse Flow Acrylics Alpine Green 2 - DAO82 Evergreen Brilliant Alizarine 2 - DA179 Alizarin Crimson 1 - DA144 Yellow Light 1 - DA159 Cherry Red Antique Blue 1 - DAO38 Wedgewood Blue Burgundy 1 - DA140 Red Violet 1 - DA166 Deep Midnight Blue 1 - DA165 Napa Red 1 - DA172 Black Plum Antique Gold 1 - DA146 Antique Gold Deep Burnt Sienna DA223 Traditional Burnt Sienna ato - DAO67 Lamp (Ebony) Black Antique Green 2 - DA105 Blue Grey Mist Burnt Umber 2 - DA221 Traditional Burnt Umber 1 - DAO84 Midnite Green 1 - DA160 Antique Maroon Antique White 2 - DA239 Warm White Cadmium Orange 8 - DA228 Bright Orange 1 - DAO3 Buttermilk 1 - DAO10 Cadmium Yellow Aqua Green Light 2 - DAO1 Snow (Titanium) White Cadmium Red Medium DAO15 Cadmium Red 1 - DAO47 Bluegrass Green Ash Pink 4 - DA164 Light Buttermilk Cadmium Yellow Light DA144 Yellow Light 3 - DA189 Summer Lilac 2 - DA186 French Mauve Aureolin Yellow 4 - DA144 Yellow Light Cadmium Yellow Medium DA227 Bright Yellow 1 - DA146 Antique Gold Deep 1 - DAO10 Cadmium Yellow Australian Olive Green 10 - DA113 Plantation Pine Carbon Black DAO67 Lamp (Ebony) Black 1 - DAO67 Lamp (Ebony) Black Australian Red Violet DA140 Red Violet Cerulean Blue DAO36 True Blue Australian Sap Green 2 - DA113 Plantation Pine Chromium Green Oxide 2 - DAO51 Leaf Green 1 - DA144 Yellow Light 1 - DAO53 Mistletoe Australian Sienna 1 - DA223 Traditional Burnt Sienna Cobalt Blue 2 - DA141 Blue Violet 1 - DA194 Marigold -

The Platinum Print: a Catalyst for Discussion
The Platinum Print: a catalyst for discussion Defined: distinguished by its matte finish and subtle tonal gradations image is embedded into the fibers of the paper non-silver process iron salts are used to sensitize paper prior to development Upon exposure to light, a faint image appears development process converts iron salts platinum faint image becomes more pronounced1 1. The History of the Platinum Print Due to the similar qualities and processes, The terms platinotype, palladium prints and platinum prints are often used interchangeably. According to James Reilly, the platinotype and the platinum print are in fact congruent photographic processes yielding in what would be the same photographic print. Having said that please refer to figure 1.1, below. This chart demonstrates not necessarily the date of invention or discovery of the most popular historic photographic processes, but rather the years in which the process was most dominantly used. 1 Figure 1.1 Chronology of the use of photographic processes in the United States. (up to 1984.) The chart indicates that platinum prints were popular for approximately 40 years from 1870s to the 1930s, competing with the well-established albumen prints and eventually being overtaken by the gelatin silver processes which came on the scene about the same time. A long string of inventors and their inventions led to the ultimate culmination in what became known as the platinum print, one of the few non-silver processes used in photography. In 1830, Ferdinand Gehlen noted that ultraviolet light would alter the color of platinum salts and cause the ferric salts to separate out into a ferrous state. -

The Platinum/Palladium Process
9 The Platinum/Palladium Process OVERVIEW AND EXPECTATIONS In the majority of the classes and workshops that I’ve taught over the years, “the platinum/palladium process” is the answer that surfaces first when I ask the question, “What process do you want to learn the most?” In this chapter you will learn how, and, as in previous chapters, I begin with a little history. Then you will learn the chemistry and sequence of the various stages to a finished print. This chapter gives you alternatives to traditional platinum/palladium chemistry and provides you with a simple sensitizer “drop chart” that is based on the type of negative you are working with, rather than the print you would like to make. I also provide the beginnings of a trouble-shooting list to assist in hunting down problems that may be showing up in your work. Finally, you’ll get some brief alternative ideas for combining platinum/palladium with other techniques such as Van Dyke and gum bichromate. A LITTLE HISTORY Like most refined non-silver and alternative photographic processes, the art of platinum/palladium printing was developed in pieces over time by a number of dedicated artists and scientists. In 1830, Ferdinand Gehlen recorded the action and effects of light on platinum chloride, noting that UV light would alter the color of platinum salts and cause the ferric salts to precipitate out into a ferrous state. At around the same time, Johann Wolfgang Dobereiner (1780–1849) observed the decomposition of ferric oxalate on exposure to UV light and scientifically defined its sensitivity. -

Nuances De Vie
NUANCES DE VIE: PHOTOGRAPHIC PRINTMAKING IN THREE MEDIUMS A Project Presented to the faculty of the Departments of Art and Design California State University, Sacramento Submitted in partial satisfaction of the requirements for the degree of MASTER OF ARTS in SPECIAL MAJOR (Printmaking and Photography) by Valerie Wheeler SPRING 2012 © 2012 Valerie Wheeler ALL RIGHTS RESERVED ii NUANCES DE VIE: PHOTOGRAPHIC PRINTMAKING IN THREE MEDIUMS A Project by Valerie Wheeler Approved by: ________________________________, Sponsor Sharmon Goff ________________________________, Committee Member Roger Vail ________________________________, Committee Member Nigel Poor _______________________ Date iii Student: Valerie Wheeler I certify that this student has met the requirements for format contained in the University format manual, and that this project is suitable for shelving in the Library and credit is to be awarded for the project. ______________________________, Dean ________________ Chevelle Newsome, Ph.D. Date Office of Graduate Studies iv Abstract of NUANCES DE VIE: PHOTOGRAPHIC PRINTMAKING IN THREE MEDIUMS by Valerie Wheeler The goal of this special major in printmaking and photography was to bridge the two art forms through photo etching using classical and modern methods. In the process of learning large format photography, intaglio printmaking (photogravure), and non-etch intagliotype printing, I expanded the project to include platinum and palladium printing (making platinotypes and platino-palladiotypes). The continuity among the mediums rested upon the images, a few of which were printed in more than one medium. Landscapes, floral still-lifes, architecture, and a few portraits came together in a body of complementary work consisting of fifty-two images in four sizes. The thesis exhibition was installed and open for a week in the Robert Else Gallery; it included short technical labels to explain the three mediums. -

Everything In
University of Texas at El Paso ScholarWorks@UTEP Open Access Theses & Dissertations 2020-01-01 Everything In Greg Chavez University of Texas at El Paso Follow this and additional works at: https://scholarworks.utep.edu/open_etd Part of the Creative Writing Commons Recommended Citation Chavez, Greg, "Everything In" (2020). Open Access Theses & Dissertations. 3149. https://scholarworks.utep.edu/open_etd/3149 This is brought to you for free and open access by ScholarWorks@UTEP. It has been accepted for inclusion in Open Access Theses & Dissertations by an authorized administrator of ScholarWorks@UTEP. For more information, please contact [email protected]. EVERYTHING IN GREG CHAVEZ Master’s Program in Creative Writing APPROVED: Sylvia Aguilar-Zéleny, MH, MFA, Chair Jeffrey Sirkin, Ph.D. Annika Mann, Ph.D. Stephen L. Crites, Jr., Ph.D. Dean of the Graduate School Copyright © by Greg Chavez 2020 EVERYTHING IN by GREG CHAVEZ, B.A. THESIS Presented to the Faculty of the Graduate School of The University of Texas at El Paso in Partial Fulfillment of the Requirements for the Degree of MASTER OF FINE ARTS Department of Creative Writing THE UNIVERSITY OF TEXAS AT EL PASO December 2020 Acknowledgements I wish to express my sincerest gratitude for the entire creative writing community at UTEP. Your invaluable feedback and encouragement along the way has helped guide my writing into and out of spaces it had feared to tread. Special thanks to all my UTEP professors who challenged me to see writing and literature from perspectives never imagined. To my thesis committee, Professor Sylvia Aguilar-Zéleny, Dr. Jeffrey Sirkin, and Dr. -

Color Chart Includes Those Colors Made from Inorganic Pigments, That Is, Metal Ores Dug from the Earth
GAMBLIN ARTISTS COLORS GAMBLIN ARTISTS OIL COLORS Artists Oil mineral inorganic colors modern organic colors Colors • All colors made from metals (Cadmium, Cobalt, Iron, etc.) are “inorganic” • Carbon based pigments are “organic” • 19th century colors of the Impressionists and the colors of Classical and Renaissance era painters • 20th century colors • High pigment load, low oil absorption • Most pigments available in a warm and cool version (ex. Phthalo Green, Phthalo Emerald) • Colors easily grey-down in mixtures, excellent for painting natural colors and light • Best choice for high key painting, bright tints • Mostly opaque with a few semi-transparent and transparent colors • Mostly transparent, with some semi-transparent colors Impressionist 20th Century CADMIUM CHARTREUSE CADMIUM LEMON CADMIUM YELLOW LIGHT CADMIUM YELLOW MEDIUM CADMIUM YELLOW DEEP HANSA YELLOW LIGHT HANSA YELLOW MEDIUM HANSA YELLOW DEEP INDIAN YELLOW CADMIUM ORANGE CADMIUM ORANGE DEEP CADMIUM RED LIGHT CADMIUM RED MEDIUM CADMIUM RED DEEP PERMANENT ORANGE TrANSPARENT OrANGE NAPTHOL RED NAPTHOL SCARLET PERYLENE RED white · grey · black ALIZARIN CrIMSON MANGANESE VIOLET COBALT VIOLET ULTRAMARINE VIOLET ALIZARIN PERMANENT QUINACRIDONE RED QUINACRIDONE MAGENTA QUINACRIDONE VIOLET DIOXAZINE PURPLE TITANIUM WHITE RADIANT WHITE TITANIUM ZINC WHITE QUICK DRY WHITE FLAKE WHITE REPLACEMENT ULTRAMARINE BLUE COBALT BLUE PrUSSIAN BLUE CERULEAN BLUE COBALT TEAL INDANTHRONE BLUE PHTHALO BLUE CERULEAN BLUE HUE MANGANESE BLUE HUE PHTHALO TURQUOISE FASTMATTE TITANIUM WHITE ZINC WHITE -

History of Communications Media
History of Communications Media Class 5 History of Communications Media • What We Will Cover Today – Photography • Last Week we just started this topic – Typewriter – Motion Pictures • The Emergence of Hollywood • Some Effects of the Feature Film Photography - Origins • Joseph Nicephore Niepce –first photograph (1825) – Used bitumen and required an 8-hour exposure – Invented photoengraving • Today’s photolithography is both a descendent of Niepce’s technique and the means by which printed circuits and computer chips are made – Partner of Louis Daguerre Photography - Origins • Louis Daguerre – invented daguerreotype – Daguerre was a panorama painter and theatrical designer – Announced the daguerreotype system in 1839 • Daguerreotype – a photograph in which the image is exposed onto a silver mirror coated with silver halide particles – The first commercially practical photographic process • Exposures of 15 minutes initially but later shortened – The polaroid of its day – capable of only a single image Photography – Origins • William Henry Fox Talbot – invented the calotype or talbotype – Calotype was a photographic system that: • Used salted paper coated with silver iodide or silver chloride that was developed with gallic acid and fixed with potassium bromide • Produced both a photographic negative and any desired number of positive prints Photography – Origins • Wet Collodion Process - 1 – Invented in 1850 by Frederick Scott Archer and Gustave Le Grey – Wet plate process that required the photographer to coat the glass plate, expose it, -

The Platinum Print B the History of the Platinum Process
THE PLATINUM PRINT B THE HISTORY OF THE PLATINUM PROCESS A REFERENCE TO THE SCIENTIFIC, COMMERCIAL, AND AESTHETIC DEVELOPMENT OF THE PLATINOTYPE JOHN HAFEY & TOM SHILLEA The Platinum Print by John Hafey and Tom Shillea ISBN 0-89938-000-X Copyright 1979 Graphic Arts Research Center Rochester Insitute of Technology This Adobe Acrobat document produced in november 2002 is based on the illustrated primer The Platinum Print written by John Hafey and Tom Shillea. It is about an exhibition called „The Contemporary Platinotype“ which took place at Rochester Institute of Technology in 1979. Unfortunately, that illustrated primer is now out of print. But the PDF-Version contains both the original text about the scientific discovery, the commercial developement and the aesthetic evolution of the Platinotype. It is requested to make use of this document for scientific research or pure information only! If any person may be offended because of misuse of copyright laws, please contact me at [email protected] Frank Rossi, 2002 Scientific Discovery and Commercial Development Ferdinand Gehlen was the first person to explore the action and effects of light rays upon platinum and record his experiments. In 1830, he discovered that a solution of platinum chloride when exposed to light, first turned a yellow color, and eventually formed a precipitate of metallic platinum.1 In 1831, experiments by the chemist Johann Wolfgang Dobereiner obtained important results. Born in Bavaria in 1780, he practiced pharmacy in Karlsruhe, and devoted himself to the study of the natural sciences, particularly chemistry. In 1810, he was given the position of professor of chemistry and pharmacy at the University of Jena, where he taught until his death in 1849.2 He observed that platinum metal was only slightly affected by the action of light, and concluded that some substance would have to be added to the pure platinum metal to in- crease its sensitivity to light. -

Platinum, Silver- Platinum, and Palladium Prints
Noble Metals for the Early Modern Era: Platinum, Silver- Platinum, and Palladium Prints Constance Mc Cabe Histories of photography usually emphasize the photogra- phers’ command of the camera and the resulting pictures while offering little insight into the extensive chemical and technical artistry performed in studios and darkrooms. Research into the materials and methods behind photo- graphic prints, however, can shed light on the aesthetic goals of the photographers and help to determine which properties are the result of artistic decisions and which might be the natural effects of aging. By studying the array of platinum, silver- platinum, and palladium prints in the Thomas Walther Collection, we are given an opportunity to appreciate how photographers in the early twentieth cen- tury manipulated materials and chemicals to achieve a quasi-modern aesthetic. The aesthetic benchmark for many photographers at the dawn of the twentieth century was the platinum print, extolled for its unparalleled artistic qualities and perma- nence. Alfred Stieglitz (1864–1946) and Clarence H. White (1871–1925), both highly influential photographers and lead- ers of Pictorialism, a movement championing photography as fine art, praised platinum as the ideal photographic medium for their exhibition prints. Their disciples contin- ued to test the medium for new and unusual effects, exploiting such curiosities as multiple exposures, tone reversal, and solarization, and exploring unconventional compositional elements and abstraction. Highly attuned to the technical craft of their work, they investigated myriad products and chemical modifications to achieve their artistic fig. 1 Alfred Stieglitz. From the Back Window at “291”. April 3, 1915. Platinum print, objectives. -

Ven in the Infancy of Their Medium, Photogra Phers Sought a Means Of
ven in the infancy of their medium, photogra A variety of cameras was designed over the next sixty phers sought a means of extending the frame of years that incorporated one of three techniques, all of E a photograph beyond its conventional propor which enabled the panoramic image to be made within tions. William Henry Fox Talbot, developer of the the camera itself: a sweeping lens and curved film calotype, the negative/positive process that he refined plane, such as the Megaskop; an extremely wide-angle, two years after he and J. L. M. Daguerre revealed to fixed lens and fixed film plane; or a fixed lens on a cam the world their different methods of photography, was era that rotates as the film is driven through a flat creating panoramic views as early as 1843. Talbot pro plane. duced his panoramas by making a sequence of overlap Until the invention of roll film in 1885, these cameras ping photographs that were displayed as pieced- were generally used only by professional photographers together sections to produce a continuous view. While or serious amateurs. Cameras such as the Multiscope early daguerreans also used this method for the same and Film Company’s Al-Vista (1898), the first popular effect, Friedrich von Martens invented the first pano panoramic camera, or Eastman Kodak’s Panoram ramic camera in 1844, the Megaskop, designed with a (1899) were easy for anyone to use, and a fascination movable lens that panned across a scene to produce a with the panoramic photograph became widespread. 150-degree view on a curved daguerreotype plate.