Contaminant Assessment and Reduction Project Final Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Waterfowl Count 2020
New York State January Waterfowl Count Report – 2020 The Ulster County segment of the annual NYSOA New York State January Waterfowl Count was conducted on January 18, 2020. Twenty participants in eight field parties encountered a remarkable 21 species and 10,012 individual waterfowl, surpassing our previous high count of 17 species recorded in 2016, 2013, and 2008. Our ten-year average for this countywide effort is 11.8 species and 6,225 individuals. Typical for this annual mid-winter survey, two species accounted for 94% of our total abundance, Canada Goose (82%) and Mallard (12%). A total of 16 Bald Eagles (9 adults and 7 sub- adults) were observed during the course of the waterfowl count. Extensive open water, exposed fields, and extremely mild air temperatures less than a week prior to the count encouraged a noticeable movement of waterfowl into the mid-Hudson River region, with air temperatures reaching the upper 60’s (F) on two consecutive days over the previous weekend. Cold arctic air eventually moved into our area mid-week and remained in place, freezing smaller and slower- moving bodies of water. This sudden change induced some waterfowl to congregate in significant areas of open water on creeks, rivers, and reservoirs, and an exceptionally good diversity of waterfowl remained in the county for count day. The highlight of this year’s survey was principally the magnitude of diversity encountered on a waterfowl count that typically tallies in the low teens. Although there was no particular outstanding species this year, Hooded Mergansers were found in record high numbers (35), surpassing our previous high count of 20, more than four times our ten-year average of 8.2/year. -

2008 Waterfowl Count Report
New York State Waterfowl Count – 2008 January 12, 2008 Ulster County Narrative Page 1 of 8 Sixteen observers in five field parties participated in the Ulster County segment of the annual New York State Winter Waterfowl Count, recording a total of 17 species and 6,890 individuals within the county on Saturday, 12 January 2008. This represents a record high species count, exceeding last year's diversity by three species, and is just 204 individuals short of our record high total set in 2006. Field observers noted fast moving water, and essentially frozen ponds, lakes, and marshes throughout the county. Stone Ridge Pond on Mill Dam Road was the exception, and continues to contribute a large number of individuals and a few unusual species to the composite, hosting American Wigeon, Ring-necked Duck, and 1,061 individuals this year. The Hudson River, Ashokan Reservoir, lower Esopus Creek in Saugerties, and agricultural fields surrounding Wallkill prison accounted for the majority of the balance of the count. Weather conditions were quite favorable for this time of the year, especially in comparison to the rain and wide- spread fog of last year, or the sub-freezing temperatures typical of a mid-January count. A very dense fog did persist over the Hudson River early morning, requiring some minor route changes to allow for early visits to inland sites while delaying surveys of the Hudson to later in the day. Temperatures started out just below freezing, then warmed to a very comfortable mid-40's (F) by afternoon. Winds were calm for the most part, with the exception of a cold NW gale sweeping across partially frozen Ashokan Reservoir, making for very choppy waters in the lower basin and difficult viewing conditions. -

Acipenser Brevirostrum
AR-405 BIOLOGICAL ASSESSMENT OF SHORTNOSE STURGEON Acipenser brevirostrum Prepared by the Shortnose Sturgeon Status Review Team for the National Marine Fisheries Service National Oceanic and Atmospheric Administration November 1, 2010 Acknowledgements i The biological review of shortnose sturgeon was conducted by a team of scientists from state and Federal natural resource agencies that manage and conduct research on shortnose sturgeon along their range of the United States east coast. This review was dependent on the expertise of this status review team and from information obtained from scientific literature and data provided by various other state and Federal agencies and individuals. In addition to the biologists who contributed to this report (noted below), the Shortnose Stugeon Status Review Team would like to acknowledge the contributions of Mary Colligan, Julie Crocker, Michael Dadswell, Kim Damon-Randall, Michael Erwin, Amanda Frick, Jeff Guyon, Robert Hoffman, Kristen Koyama, Christine Lipsky, Sarah Laporte, Sean McDermott, Steve Mierzykowski, Wesley Patrick, Pat Scida, Tim Sheehan, and Mary Tshikaya. The Status Review Team would also like to thank the peer reviewers, Dr. Mark Bain, Dr. Matthew Litvak, Dr. David Secor, and Dr. John Waldman for their helpful comments and suggestions. Finally, the SRT is indebted to Jessica Pruden who greatly assisted the team in finding the energy to finalize the review – her continued support and encouragement was invaluable. Due to some of the similarities between shortnose and Atlantic sturgeon life history strategies, this document includes text that was taken directly from the 2007 Atlantic Sturgeon Status Review Report (ASSRT 2007), with consent from the authors, to expedite the writing process. -

Frequently Asked Questions (FAQ)
Town of Esopus Local Waterfront Revitalization Project (LWRP) Frequently Asked Questions . What is an LWRP? New York State's Local Waterfront Revitalization Program (LWRP) is a comprehensive framework for future land and water uses prepared by local communities in partnership with the Department of State. Similar to a comprehensive plan, it outlines a vision for the future growth and management of a community, with a specific focus on the waterfront lands. The Town of Esopus LWRP refines and supplements the State’s Coastal Management Program. Once an LWRP is officially adopted by a local government, it becomes a standard by which future land and water use decisions-such as zoning changes or development proposals-should comply with. Communities which have an approved LWRP are eligible to apply for grant funding to work toward their waterfront vision. For more information, see the Department of State LWRP guidance page. What is the goal of this community effort? The goal is to develop a comprehensive framework for the long-term use of the waterfront which will strive to do the following: help maintain and protect water quality; protect the natural environment; enhance public access to the river; provide new recreational opportunities; restore and revitalize former industrial land on the water; and stimulate economic development in the Town of Esopus. How is this different from the Esopus Riverfront Access and Connections Study? The recently completed Esopus Riverfront Access and Connections Study (the "Study"), which was funded by the New York State Department of Environmental Conservation Hudson River Estuary Program, focused on improving recreational waterfront access on the Hudson River and estuary section of the Rondout Creek. -

New York State January Waterfowl Count Report – 2018 the Ulster County Segment of the Annual NYSOA New York State January Wate
New York State January Waterfowl Count Report – 2018 The Ulster County segment of the annual NYSOA New York State January Waterfowl Count was conducted on January 13, 2018. Considerable ice, raging water, and flooded fields presented challenges and opportunities for the seventeen participants in six field parties as we collectively tallied 2,041 individuals representing 13 species during 8.5 hours of effort (8:00 a.m. - 4:30 p.m.). Diversity was close to average (12.1 ten-year average); however, abundance was well below our ten-year average of 6,244 and a new record low for the fourteen-year period that I have compiled this count. Perhaps not surprisingly, this year’s count was in stark contrast to last year’s remarkable tally of 13,500 individuals under more hospitable environmental conditions. Two weeks of consistently frigid air temperatures solidified most bodies of water, followed by a very brief warm-up with heavy rain just prior to the count, setting the stage for a count day featuring substantial ice with rapid and high water flows in turbulent channels. Count day temperatures ranged from a morning high of 32° (F), dropping to an afternoon low of 29° (F). Early morning rain and fog gave way to overcast skies by 9:00 a.m. and eventually cleared somewhat, with partly sunny skies by mid-day. Winds were generally calm to 10-15 mph. Most of the Hudson River was covered in ice and largely devoid of waterfowl. Kingston Point produced twenty Mallards and eight American Black Ducks, and a few Common Mergansers were found off Rider Park in the Town of Ulster and in a small area of open water farther south at Mariner's Restaurant in Highland. -

Preliminary 70X30 Scenario Pocket
? ? I R A TO TO T N 2 ROBERT MOSES/ST. LAWRENCE O O ! 1 / 3 3 / REYNOLDS MASON CORNERS / ROUSES ALCOA POINT Massena ● 2 CHATEAUGAY / MARBLE 0+/ CHATEAUGAY RIVER ? ? / -/ SCIOTA MASSENA / -/ PATNODE FLAT ROCK WILLIS / DENNISON JERICHO RISE / ALTONA MACOMB ! /- ! RAYMONDVILLE ● RYAN -/ MALONE / Lower ● DULEY BRADY 2 ! NORFOLK Chateaugay -/ CLINTON ? Lake ? -/ ● ! EAST NORFOLK ELLENBURG ASHLEY ROAD YALEVILLE ! 2 / Chazy NORWOOD ! LAWRENCE SARANAC ENERGY UNIONVILLE & ! AVE Lake LYON MTN. / Lake PLATTSBURGH / Ogdensburg Titus / X HEWITTVILLE / 0+ ALLENS ● NICHOLVILLE Upper Dannemora N. OGDENSBURG / Potsdam Chateaugay / / FALLS Lake ● MCINTYRE SUGAR ISLAND ! PLATTSBURG H MUNICIPAL SANDBAR HANNAWA KENTS FALLS/ ! ! DeerRiver / Canton LITTLE / PARISHVILLE Flow SARANAC NORTHEND RIVER ? ? ! COLTON ● 2 Lake 2 Ozonia C L I N T O N MCADOO / 2 Meacham FIVEFALLS Lake e PYRITES / HIGLEY ! UNION Lak Ontario ! ! RAINBOW DEKALB / Warm Brook F R A N K L I N Rainbow Union / SOUTH ! Silver Lake HALLOCK Black Flow ! ! BLAKE Lake Falls COLTON Pond HILL Lake Blake Falls Reservoir NINE MILE #1 Taylor Lake INDEPENDENCE NINE MILE #2 ! STARK Osgood Pond Champlain 0' 2 Stark Falls Pond FRANKLIN ! HAMMERMILL ALCAN 0+ '0 J.A. FITZPATRICK Reservoir OSWEGO '0 2 Lake )/ Clear 0% 2 NORTH Carry Falls 2 WINE Reservoir ● LAKE COLBY ! CREEK SCRIBA GOUVERNEUR / BATTLE 2 Upper Saranac PALOMA 2 Butterfield ● HILL Lower Saranac Lake SOUTH Lake Gouverneur Lake VARICK 2 Yellow Wolf Lake Saranac ! Mud S T . L A W R E N C E Pond Lake Placid OSWEGO HIGHDAM Lake Lake Oseetah Red Lake ● Lake Placid 3 Lake / MINETTO 2 FLAT ROCK NEWTON Piercefield LAKE ! ● THOUSAND BALMAT 2 Chaumont Moon FALLS Flow Middle PLACID BARTON BROOK ISLANDS ! Pond / 2 Lake / PIERCEFIELD ! TupperLake Saranac ! Lake Tupper BROWNS Follensby Lake VOLNEY FALLS Simon Pond Pond Lincoln FULTON J E F F E R S O N Pond CCOOPPEENNHHAGAEGNEWNINWDIND ! BLACK /- Lake Cranberry O S W E G O FORT Bonaparte Lake E S S E X Fulton / PercRhIVER ! BRISTOL DRUM / LYME Lake / ST. -

Alliance Annual Report 2016
wallkill river DRAFT watershed alliance we fight dirty ANNUAL REPORT 2016 Some of the fourteen members of the July 14th Boat Brigade from New Paltz to Rosendale. 2017 Board of Directors Jason West, President Dan Shapley, Treasurer Neil Bettez Arthur Cemelli Jillian Decker Richard Picone Michael Sturm 2016 Working Group Chairs Brenda Bowers, Boat Brigades Archie Morris, Boat Brigades Rob Ferri, Boat Brigades Martha Cheo, Outreach & Policy Working Group Neil Bettez, Science Working Group This annual report has been funded in part by a grant from the New York State Environmental Protection Fund through the Hudson River Estuary Program of the New York State Department of Environmental Conservation. !1 of 22! wallkill river DRAFT watershed alliance we fight dirty TABLE OF CONTENTS Introduction……………………………… 2 Water Quality……………………………. 3 GIS…………………………………….. 4 Nutrient Testing Program……………… 4 Harmful Algae Bloom…………………. 5 WAVE Trainings……………………….. 6 Public Access and Engagement………….. 7 Boat Brigades………………………….. 8 Tabling and Online Outreach………….. 8 Educational Program………………….. 8 Website and Videos……………………. 9 Capacity Building………………………… 9 Governance……………………………. 9 Finances………………………………..10 Grants………………………………. …10 Non-Alliance Wallkill Projects…………….11 Appendix A: Harmful Algal Bloom Timeline and Sampling Results…….. Appendix B: Detailed WAVE Results……. The crew of the Alliance’s Happy Heron in the Regatta Parade. Appendix C: 2016 Budget……………….. New Paltz, May 1st. (l-r) Craig Chapman, Archie Morris, Arthur Cemelli, and Rich Picone. Appendix D: Boat Brigade Reports……… _________________________________________________________ INTRODUCTION The Wallkill River Watershed Alliance was founded just eighteen months ago, making 2016 has been the first full year for the Alliance. This first Annual Report to the membership and Board of Directors highlights the most important developments within the Alliance over the past year. -
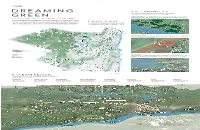
Set Priorities . Make a Map . Create Results
GREEN SPACES SUPP_MM8494_B [P];77.indd 1 .SET PRIORITIES D R E A M I N G The map helps clarify conservation priorities, which vary from state to state and county to county. It also helps planners target sites where two goals can be accomplished at once—where a new wildlife corridor, for example, can double as a hiking trail. Connected habitats Preventing the fragmentation of wildlife habitat is a priority in Ulster. In June 2015 GREEN the nonprofi t group Scenic Hudson preserved a 23-acre mix of forest and hardwood swamp, creating the John Burroughs Black Creek Corridor and fi lling a gap in a wildlife pathway. Blueback herring can now migrate more CASE STUDY: ULSTER COUNTY, NEW YORK safely, and people can hike and paddle along the eight-mile-long corridor. Just a hundred miles north of Manhattan, Ulster is a mostly rural county that spans the Catskill J o h Chodikee n B u r r o Mountains and the Hudson River Valley. Long a source of New York City’s drinking water, it also Lake u g h s . MAKE A MAP B has become a booming market for tourism and second homes. Ulster’s challenge is a common BBLACKLACK CCREEKREEK B l a la c STATE FOREST ck k one: how to encourage development while protecting natural habitat and quality of life. In 2009, Ulster County offi cials evaluated land according to several criteria—look- Cr C ee ing fi rst at the ecology of the landscape, then at other factors, including JOHN k r advised by the nonprofi t Green Infrastructure Center, a new county government embarked on a e its historical signifi cance and its value as working farmland—to create BURROUGHS e NATURE k three-step process to create—and act on—a “greenprint” for conservation and development. -

Waterfowl Count 2015
New York State January Waterfowl Count – January 17, 2015 Fourteen observers in seven field parties participated in the 2015 Ulster County segment of the annual NYSOA New York State January Waterfowl Count on Saturday, 17 January, encountering a total of 4,351 individuals of 8 species during an 8-hour effort. Waterfowl were generally scarce and in low numbers, relative to this mid-winter count that often presents challenges to locating open water and ducks, geese, or swans. The final tally tied our 2011 effort for the lowest number of species recorded over the past ten years, and individual abundance was well below our ten-year average of 5,515 birds/year, and less than half our high count of 8,874 in 2012. All of our field parties reported extensive areas of frozen water and a general lack of waterfowl, with the stand-out exception of the Wallkill River in the southern part of the county where a nice diversity and abundance of birds was encountered in several locations. The Wallkill Valley sector, including Blue Chip Farm, produced our greatest diversity and more individuals than all of our other sectors combined, emphasizing just how scarce waterfowl were throughout the rest of the county. Count day visibility was excellent, with bright sunny skies, no precipitation and no wind. Below freezing temperatures (11-24 degrees F) and significant areas of frozen water resulted in virtually no fog or heat wave interference, but also significantly reduced the amount of viable waterfowl habitat and limited open water in the Hudson River to a very narrow channel, where a combination of Coast Guard and barge activity, distance, and large blocks of obstructing ice made detection of waterfowl ineffective. -

Wallkill River Watershed Conservation
Wallkill Watershed Conservation and Management Plan Page 2 Cover painting by: Gene Bové Wallkill River School www.WallkillRiverSchool.com Wallkill Watershed Conservation and Management Plan Page 3 Project Steering Committee James Beaumont Orange County Water Authority Kris Breitenfeld Orange County Soil and Water Conservation District Gary Capella Ulster County Soil and Water Conservation District Virginia Craft Ulster County Planning Department Scott Cuppett NYSDEC Estuary Program Leonard DeBuck Farmer, Warwick Town Councilman Kelly Dobbins Orange County Planning Department John Gebhards Orange County Land Trust Patricia Henighan Wallkill River Task Force Jill Knapp Wallkill River Task Force Lewis Lain Town Supervisor, Minisink Nick Miller Biologist, Metropolitan Conservation Alliance Nathaniel Sadjek NJ Wallkill River Management Group Karen Schneller-McDonald Biologist, Hickory Creek Consulting William Tully Orange County Citizens Foundation, Chester Town Supervisor James Ullrich Environmental Scientist, Alpine Group Ed Sims Orange County Health Department Kevin Sumner Orange County Soil and Water Conservation District Jake Wedemeyer Ulster County Soil and Water Conservation District Chip Watson Orange County Horse Council/NY Horse Council Alan Dumas Ulster County Health Department GIS/Mapping Committee Kelly Dobbins Orange County Planning Department Jake Wedemeyer Ulster County Soil and Water Conservation District Daniel Munoz Orange County Water Authority/Orange County Dept. of Information Services Kevin Sumner Orange County Soil -
Ulster Orange Greene Dutchess Albany Columbia Schoharie
Barriers to Migratory Fish in the Hudson River Estuary Watershed, New York State Minden Glen Hoosick Florida Canajoharie Glenville Halfmoon Pittstown S a r a t o g a Schaghticoke Clifton Park Root Charleston S c h e n e c t a d y Rotterdam Frost Pond Dam Waterford Schenectady Zeno Farm Pond Dam Niskayuna Cherry Valley M o n t g o m e r y Duanesburg Reservoir Dam Princetown Fessenden Pond Dam Long Pond Dam Shaver Pond Dam Mill Pond Dam Petersburgh Duanesburg Hudson Wildlife Marsh DamSecond Pond Dam Cohoes Lake Elizabeth Dam Sharon Quacken Kill Reservoir DamUnnamed Lent Wildlife Pond Dam Delanson Reservoir Dam Masick Dam Grafton Lee Wildlife Marsh Dam Brunswick Martin Dunham Reservoir Dam Collins Pond Dam Troy Lock & Dam #1 Duane Lake Dam Green Island Cranberry Pond Dam Carlisle Esperance Watervliet Middle DamWatervliet Upper Dam Colonie Watervliet Lower Dam Forest Lake Dam Troy Morris Bardack Dam Wager Dam Schuyler Meadows Club Dam Lake Ridge Dam Beresford Pond Dam Watervliet rapids Ida Lake Dam 8-A Dyken Pond Dam Schuyler Meadows Dam Mt Ida Falls Dam Altamont Metal Dam Roseboom Watervliet Reservoir Dam Smarts Pond Dam dam Camp Fire Girls DamUnnamed dam Albia Dam Guilderland Glass Pond Dam spillway Wynants Kill Walter Kersch Dam Seward Rensselaer Lake Dam Harris Dam Albia Ice Pond Dam Altamont Main Reservoir Dam West Albany Storm Retention Dam & Dike 7-E 7-F Altamont Reservoir Dam I-90 Dam Sage Estates Dam Poestenkill Knox Waldens Pond DamBecker Lake Dam Pollard Pond Dam Loudonville Reservoir Dam John Finn Pond Dam Cobleskill Albany Country Club Pond Dam O t s e g o Schoharie Tivoli Lake Dam 7-A . -

An Interim Watershed Management Plan for the Lower, Non-Tidal Portion of the Rondout Creek, Ulster County, New York
An Interim Watershed Management Plan for the Lower, Non-Tidal Portion of the Rondout Creek, Ulster County, New York December 2010 Prepared by the Rondout Creek Watershed Council Financial support for this document was provided by: Hudson River Estuary Program of the New York State Department of Environmental Conservation and the New England Interstate Water Pollution Control Commission An Interim Watershed Management Plan for the Lower Non-Tidal Portion of the Rondout Creek, Ulster County, New York December 2010 Prepared by the Rondout Creek Watershed Council Primary Authors: Victor-Pierre Melendez, Rondout Creek Watershed Council Coordinator, Hudson River Sloop Clearwater Jen Rubbo, Environmental Action Educator, Hudson River Sloop Clearwater Manna Jo Greene, Environmental Director, Hudson River Sloop Clearwater. Section Authors : Laura Finestone, Rochester Environmental Conservation Committee (RCWC Mission and Vision) Mary McNamara, Lower Esopus Watershed Partnership (Esopus Watershed Appendix) Katherine Beinkafner, PhD, CPG, Mid-Hudson Geosciences (Soil and Geology) Laura Heady, Hudson River Estuary Program and Cornell University (Biodiversity) Jennifer Grieser, Department of Environmental Protection (Riparian Buffers) Kevin Grieser, Department of Environmental Conservation (Riparian Buffers) Ryan Trapani, Catskill Forestry Association (Forestry and Agriculture) Martha Cheo, Hudson Basin River Watch (Water Quality) Simon Gruber, Hudson Valley Regional Council (Stormwater and Wastewater) Intern Research Assistants : Jessica Gray, Marist