Estimation of Work of Breathing from Respiratory Muscle Activity in Spontaneous Ventilation: a Pilot Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Airway Pressures and Volutrauma
Airway Pressures and Volutrauma Airway Pressures and Volutrauma: Is Measuring Tracheal Pressure Worth the Hassle? Monitoring airway pressures during mechanical ventilation is a standard of care.1 Sequential recording of airway pressures not only provides information regarding changes in pulmonary impedance but also allows safety parameters to be set. Safety parameters include high- and low-pressure alarms during positive pressure breaths and disconnect alarms. These standards are, of course, based on our experience with volume control ventilation in adults. During pressure control ventilation, monitoring airway pressures remains important, but volume monitoring and alarms are also required. Airway pressures and work of breathing are also important components of derived variables, including airway resistance, static compliance, dynamic compliance, and intrinsic positive end-expiratory pressure (auto-PEEP), measured at the bedside.2 The requisite pressures for these variables include peak inspiratory pressure, inspiratory plateau pressure, expiratory plateau pressure, and change in airway pressure within a breath. Plateau pressures should be measured at periods of zero flow during both volume control and pressure control ventilation. Change in airway pressure should be measured relative to change in volume delivery to the lung (pressure-volume loop) to elucidate work of breathing. See the related study on Page 1179. Evidence that mechanical ventilation can cause and exacerbate acute lung injury has been steadily mounting.3-5 While most of this evidence has originated from laboratory animal studies, recent clinical reports appear to support this concept.6,7 Traditionally, ventilator-induced lung injury brings to mind the clinical picture of tension pneumothorax. Barotrauma (from the root word baro, which means pressure) is typically associated with excessive airway pressures. -

Clinical Management of Severe Acute Respiratory Infections When Novel Coronavirus Is Suspected: What to Do and What Not to Do
INTERIM GUIDANCE DOCUMENT Clinical management of severe acute respiratory infections when novel coronavirus is suspected: What to do and what not to do Introduction 2 Section 1. Early recognition and management 3 Section 2. Management of severe respiratory distress, hypoxemia and ARDS 6 Section 3. Management of septic shock 8 Section 4. Prevention of complications 9 References 10 Acknowledgements 12 Introduction The emergence of novel coronavirus in 2012 (see http://www.who.int/csr/disease/coronavirus_infections/en/index. html for the latest updates) has presented challenges for clinical management. Pneumonia has been the most common clinical presentation; five patients developed Acute Respira- tory Distress Syndrome (ARDS). Renal failure, pericarditis and disseminated intravascular coagulation (DIC) have also occurred. Our knowledge of the clinical features of coronavirus infection is limited and no virus-specific preven- tion or treatment (e.g. vaccine or antiviral drugs) is available. Thus, this interim guidance document aims to help clinicians with supportive management of patients who have acute respiratory failure and septic shock as a consequence of severe infection. Because other complications have been seen (renal failure, pericarditis, DIC, as above) clinicians should monitor for the development of these and other complications of severe infection and treat them according to local management guidelines. As all confirmed cases reported to date have occurred in adults, this document focuses on the care of adolescents and adults. Paediatric considerations will be added later. This document will be updated as more information becomes available and after the revised Surviving Sepsis Campaign Guidelines are published later this year (1). This document is for clinicians taking care of critically ill patients with severe acute respiratory infec- tion (SARI). -

Respiratory Therapy Pocket Reference
Pulmonary Physiology Volume Control Pressure Control Pressure Support Respiratory Therapy “AC” Assist Control; AC-VC, ~CMV (controlled mandatory Measure of static lung compliance. If in AC-VC, perform a.k.a. a.k.a. AC-PC; Assist Control Pressure Control; ~CMV-PC a.k.a PS (~BiPAP). Spontaneous: Pressure-present inspiratory pause (when there is no flow, there is no effect ventilation = all modes with RR and fixed Ti) PPlateau of Resistance; Pplat@Palv); or set Pause Time ~0.5s; RR, Pinsp, PEEP, FiO2, Flow Trigger, rise time, I:E (set Pocket Reference RR, Vt, PEEP, FiO2, Flow Trigger, Flow pattern, I:E (either Settings Pinsp, PEEP, FiO2, Flow Trigger, Rise time Target: < 30, Optimal: ~ 25 Settings directly or by inspiratory time Ti) Settings directly or via peak flow, Ti settings) Decreasing Ramp (potentially more physiologic) PIP: Total inspiratory work by vent; Reflects resistance & - Decreasing Ramp (potentially more physiologic) Card design by Respiratory care providers from: Square wave/constant vs Decreasing Ramp (potentially Flow Determined by: 1) PS level, 2) R, Rise Time ( rise time ® PPeak inspiratory compliance; Normal ~20 cmH20 (@8cc/kg and adult ETT); - Peak Flow determined by 1) Pinsp level, 2) R, 3)Ti (shorter Flow more physiologic) ¯ peak flow and 3.) pt effort Resp failure 30-40 (low VT use); Concern if >40. Flow = more flow), 4) pressure rise time (¯ Rise Time ® Peak v 0.9 Flow), 5) pt effort ( effort ® peak flow) Pplat-PEEP: tidal stress (lung injury & mortality risk). Target Determined by set RR, Vt, & Flow Pattern (i.e. for any set I:E Determined by patient effort & flow termination (“Esens” – PDriving peak flow, Square (¯ Ti) & Ramp ( Ti); Normal Ti: 1-1.5s; see below “Breath Termination”) < 15 cmH2O. -

Near Drowning
Near Drowning McHenry Western Lake County EMS Definition • Near drowning means the person almost died from not being able to breathe under water. Near Drownings • Defined as: Survival of Victim for more than 24* following submission in a fluid medium. • Leading cause of death in children 1-4 years of age. • Second leading cause of death in children 1-14 years of age. • 85 % are caused from falls into pools or natural bodies of water. • Male/Female ratio is 4-1 Near Drowning • Submersion injury occurs when a person is submerged in water, attempts to breathe, and either aspirates water (wet) or has laryngospasm (dry). Response • If a person has been rescued from a near drowning situation, quick first aid and medical attention are extremely important. Statistics • 6,000 to 8,000 people drown each year. Most of them are within a short distance of shore. • A person who is drowning can not shout for help. • Watch for uneven swimming motions that indicate swimmer is getting tired Statistics • Children can drown in only a few inches of water. • Suspect an accident if you see someone fully clothed • If the person is a cold water drowning, you may be able to revive them. Near Drowning Risk Factor by Age 600 500 400 300 Male Female 200 100 0 0-4 yr 5-9 yr 10-14 yr 15-19 Ref: Paul A. Checchia, MD - Loma Linda University Children’s Hospital Near Drowning • “Tragically 90% of all fatal submersion incidents occur within ten yards of safety.” Robinson, Ped Emer Care; 1987 Causes • Leaving small children unattended around bath tubs and pools • Drinking -

The Future of Mechanical Ventilation: Lessons from the Present and the Past Luciano Gattinoni1*, John J
Gattinoni et al. Critical Care (2017) 21:183 DOI 10.1186/s13054-017-1750-x REVIEW Open Access The future of mechanical ventilation: lessons from the present and the past Luciano Gattinoni1*, John J. Marini2, Francesca Collino1, Giorgia Maiolo1, Francesca Rapetti1, Tommaso Tonetti1, Francesco Vasques1 and Michael Quintel1 Abstract The adverse effects of mechanical ventilation in acute respiratory distress syndrome (ARDS) arise from two main causes: unphysiological increases of transpulmonary pressure and unphysiological increases/decreases of pleural pressure during positive or negative pressure ventilation. The transpulmonary pressure-related side effects primarily account for ventilator-induced lung injury (VILI) while the pleural pressure-related side effects primarily account for hemodynamic alterations. The changes of transpulmonary pressure and pleural pressure resulting from a given applied driving pressure depend on the relative elastances of the lung and chest wall. The term ‘volutrauma’ should refer to excessive strain, while ‘barotrauma’ should refer to excessive stress. Strains exceeding 1.5, corresponding to a stress above ~20 cmH2O in humans, are severely damaging in experimental animals. Apart from high tidal volumes and high transpulmonary pressures, the respiratory rate and inspiratory flow may also play roles in the genesis of VILI. We do not know which fraction of mortality is attributable to VILI with ventilation comparable to that reported in recent clinical practice surveys (tidal volume ~7.5 ml/kg, positive end-expiratory pressure (PEEP) ~8 cmH2O, rate ~20 bpm, associated mortality ~35%). Therefore, a more complete and individually personalized understanding of ARDS lung mechanics and its interaction with the ventilator is needed to improve future care. -

Simulator Measurement of the Work of Breathing for Underwater
SIMULATOP REASUREBEMT OF THE RORK OF BRIiAPHING FOB UNDEEWATER BREATHING APPARATUS Durcan Moir fliln~ E. Sc. Mar ire Science Stockton stat^ Colfege, New Jers~y,1973 A THESIS SUBEITTED IN PARTIAL PULPILLEEBT OF THE PEQUIREHENTS FOR THE DEGREE OF MASTER OF SCIENCE (KfNES IOLOGY) in the Department of Kicesiology a GUNCAN ROIB BILNE 1977 SIBOH FRASER UNIVERSITY December, 1977 All rights reserved, This thesis may nct t>f reproduced in whole or ir part, by photocopy or cthor means, without permission of tho author. NAYE: Duncan noir flllni TITLE OF THESIS: Hespiration Simulator' Htlasur+m~n* , of the Wark of Bre3thi11y far Und2rwater Breathinq A ppara tus EXhnINING COMnITTEE: Chairman: Dr. E. W. R;inister 'DL. J. n. aorrison Senior Sup:.r visor Dr, T, W. ialv.?rt Dr. A. E. Zhaprssry Ext +rnal Examin~r Prof -.ssor SLlncn Praser IJniv~rsity - at A,,,,,.,: .....................Jet, , V7r PART lAL COPYR lGHT L lCENSE I hereby grant to Simon Fraser University the right to lend my thesis, project or extended essay (the title of which is shown below) to users of the Simon Fraser University Library, and to make partial or single copies only for such users or in response to a request from the library of any other university, or other educational institution, on its own behalf or for one of its users. I further agree that permission for multiple copying of this work for scholarly purposes may be granted by me or the Dean of Graduate Studies. It is understood that copying or publication of this work for financial gain shall not be allowed without my written permission. -

The Pathophysiology of 'Happy' Hypoxemia in COVID-19
Dhont et al. Respiratory Research (2020) 21:198 https://doi.org/10.1186/s12931-020-01462-5 REVIEW Open Access The pathophysiology of ‘happy’ hypoxemia in COVID-19 Sebastiaan Dhont1* , Eric Derom1,2, Eva Van Braeckel1,2, Pieter Depuydt1,3 and Bart N. Lambrecht1,2,4 Abstract The novel coronavirus disease 2019 (COVID-19) pandemic is a global crisis, challenging healthcare systems worldwide. Many patients present with a remarkable disconnect in rest between profound hypoxemia yet without proportional signs of respiratory distress (i.e. happy hypoxemia) and rapid deterioration can occur. This particular clinical presentation in COVID-19 patients contrasts with the experience of physicians usually treating critically ill patients in respiratory failure and ensuring timely referral to the intensive care unit can, therefore, be challenging. A thorough understanding of the pathophysiological determinants of respiratory drive and hypoxemia may promote a more complete comprehension of a patient’sclinical presentation and management. Preserved oxygen saturation despite low partial pressure of oxygen in arterial blood samples occur, due to leftward shift of the oxyhemoglobin dissociation curve induced by hypoxemia-driven hyperventilation as well as possible direct viral interactions with hemoglobin. Ventilation-perfusion mismatch, ranging from shunts to alveolar dead space ventilation, is the central hallmark and offers various therapeutic targets. Keywords: COVID-19, SARS-CoV-2, Respiratory failure, Hypoxemia, Dyspnea, Gas exchange Take home message COVID-19, little is known about its impact on lung This review describes the pathophysiological abnormal- pathophysiology. COVID-19 has a wide spectrum of ities in COVID-19 that might explain the disconnect be- clinical severity, data classifies cases as mild (81%), se- tween the severity of hypoxemia and the relatively mild vere (14%), or critical (5%) [1–3]. -

PALS Testing Case Scenarios
Testing Case Scenario 1 Hypovolemic Shock (Child) Scenario Lead-in Prehospital: You have been dispatched to transport a 5 year old with a 3-day history of fever Vital Signs and diarrhea. She has been increasingly lethargic in the last 2 hours. Heart rate 140/min ED: You are asked to assess and manage a 5 year old with a 3-day history of fever and Blood pressure 86/52 mm Hg diarrhea. She has been increasingly lethargic in the last 2 hours. Efforts for a peripheral Respiratory rate 36/min intravenous access have been unsuccessful. SpO2 97% on room air General inpatient unit: You are called to assess a 5 year old who has been admitted to the Temperature 38.0°C (100.4°F) ward with a 3-day history of fever and diarrhea. She has been increasingly lethargic in the last Weight 21 kg hour and has had severe ongoing diarrhea. Her intravenous access is no longer functioning. Age 5 years ICU: You are called to the bedside of a 5 year old who has been admitted to the intensive care unit with a 3-day history of fever and diarrhea. She has been increasingly lethargic in the last 2 hours and has had severe ongoing diarrhea. Her intravenous access is no longer functioning. Scenario overview and learning objectives Scenario Overview Emphasis in this scenario should be on identification of compensated hypovolemic shock. Priorities include oxygen, immediate establishment of intravenous (IV) access, and administration of fluid bolus of isotonic crystalloid, repeated as needed to treat shock signs. -

MEDICAL EQUIPMENT for MULTIPLACE HYPERBARIC CHAMBERS Part II: Ventilators
European Journal of Underwater and Hyperbaric Medicine, ISSN: 1605-9204 Volume 7 No.1, March 2006 Review Articles MEDICAL EQUIPMENT FOR MULTIPLACE HYPERBARIC CHAMBERS Part II: Ventilators Jacek Kot National Centre for Hyperbaric Medicine, Institute of Maritime and Tropical Medicine, Gdynia, Medical University of Gdansk, Poland Kot J: Medical equipment for multiplace hyperbaric chambers. Part II: Ventilators. Europ J Underwater Hyperbaric Med 2006, 7(1): 9- 12. All medical devices introduced into the hyperbaric chamber should be of an appropriate design and fit for use in the hyperbaric environment and the manufacturer should certify them for hyperbaric conditions. However, until now only several medical devices are CE marked for usage in hyperbaric chambers. Therefore users often need to perform themselves checking of the medical equipment needed for continuation of intensive care during hyperbaric treatment. To make this task easier, this paper presents review of reports of usage of medical devices under increased pressure. Part 1 concerned devices for monitoring and cardiac support. Part 2 describes mechanical ventilators and Part 3 will be devoted to infusion pumps and syringes. Hyperbaric Oxygen Therapy, Medical Equipment, CE Marking INTRODUCTION combined into Reynold’s number (Re). The Reynold’s In Part 1 of this paper (1) the review concerning number is a dimensionless value which can be calculated monitoring devices and cardiac support was presented. using equation: This Part describes mechanical ventilators. Part 3 will be density devoted to infusion pumps and syringes. Re = velocity × diameter × viscosity VENTILATORS If Re is less than 2000 than the flow will likely be According to indications accepted for HBO therapy some laminar. -

Pediatric Smoke Inhalation Injury
San Mateo County Emergency Medical Services Pediatric Smoke Inhalation Injury For patients with smoke inhalation History Signs and Symptoms Differential • Number and severity of other victims • Facial burns, pain, and/or swelling • Foreign Body Aspiration • Industrial or residential fire • Cherry red skin • Asthma exacerbation • Duration of inhalation • Loss of consciousness • Cyanide poisoning • Social history - smoking • Hypotension/shock • Carbon monoxide poisoning • Past medical history • Airway compromise/distress could be indicated by • Thermal injury • Other trauma hoarseness/wheezing • Heart failure • Odor • Seizure/AMS after industrial or closed space fire • Acute respiratory distress syndrome consider cyanide poisoning Scene safety. Quantify and Approved triage patients. Load and go Burn Receiving Centers with assessment and treatment Assess enroute to hospital St. Francis – San Francisco Airway Valley Med. Center – San Jose UC Davis – Sacramento No or Mild Airway Involvement Moderate Airway Involvement Severe Airway Involvement Airway patent, no signs of edema, no Suspected inhalation injury with only one Accessory muscle use or altered breath stridor or change in voice, nasal hairs of the following: Wheezing, change in sounds intact, low likelihood of airway voice, increased work of breathing/ OR involvement tachypnea. Any combination of the following: Airway edema, stridor, change in voice, increased work of breathing/tachypnea. If oxygen saturation ≥ 92% Monitor and reassess Routine Medical Care E Apply Oxygen to maintain goal Monitor and reassess SpO > 92% 2 E High flow Oxygen Cardiac monitor Regardless of SpO2 Cardiac monitor Consider, IV P Head Trauma Consider, Albuterol Consider, 12-Lead ECG Use Broselow Tape; refer to dosing guide Consider, IV/IO Pain Albuterol P Use Broselow Tape; refer to dosing guide Burns Notify receiving facility. -

The Physiology of Compressed Gas Diving
THE PHYSIOLOGY OF COMPRESSED GAS DIVING Simon Mitchell INTRODUCTION The breathing of compressed gas while immersed and exposed to increased ambient pressure imposes significant homeostatic challenges on the body. This chapter discusses the important mechanisms of these challenges, with particular reference to the respiratory system. RESPIRATORY SYSTEM Aspects of compressed gas breathing equipment and its function. SCUBA diving equipment is the most commonly used compressed gas system in civilian diving and illustrates the important features and function relevant to diving physiology. Basic SCUBA equipment consists of a cylinder of air at high pressure, a demand valve regulator, and a device for holding this equipment on the diver's back. In the modern context the latter is usually an inflatable jacket called a buoyancy control device (BCD) whose dual function it is to allow buoyancy adjustment in-water and carriage of the tank and regulator. Together with the wetsuit necessary for temperate water diving and weightbelt, this apparatus may constitute a significantly restrictive force over the diver's chest and abdomen. The regulator reduces the cylinder high pressure air to ambient pressure and supplies air on demand. Thus, at a depth of 30m where the absolute pressure is 4 bars, the regulator supplies air at 4 bars and the air is 4 times as dense as air at sea level (1 bar). The ambient pressure is "measured" by the regulator second stage (attached to the mouthpiece) which, in the upright diver, is approximately 20cm above the centre of the chest. The water pressure acting on the chest will therefore be approximately 20cm H2O higher than that of the inspired gas, creating a negative transmural pressure which is greatest at the lung bases. -
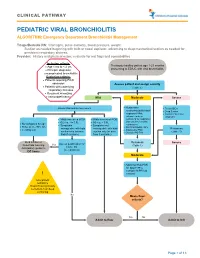
PEDIATRIC VIRAL BRONCHIOLITIS ALGORITHM: Emergency Department Bronchiolitis Management
CLINICAL PATHWAY PEDIATRIC VIRAL BRONCHIOLITIS ALGORITHM: Emergency Department Bronchiolitis Management Triage/Bedside RN: Vital signs, pulse oximetry, blood pressure, weight. Suction as needed beginning with bulb or nasal aspirator, advancing to deep/mechanical suction as needed for persistent respiratory distress. Provider: History and physical exam, evaluate for red flags and comorbidities Inclusion criteria: • Age 1 mo to < 2 yrs Previously healthy patient age 1-23 months • Principle diagnosis: presenting to ED/UC with viral bronchiolitis uncomplicated bronchiolitis Exclusion criteria: • Patients requiring PICU admission Assess patient and assign severity • Patients with underlying (Table 1) respiratory illnesses • Recurrent wheezing • Immunodeficiency Mild Moderate Severe • Assess WOB and O2 requirement Noninvasive • Treat ABCs suctioning (bulb/nasal • Deep Suction aspirator) PRN; • Consider alternative advance to deep diagnosis • Mildly increased WOB • Mildly increased WOB suctioning for respiratory • O2 req. </= 0.5L • O2 req. > 0.5L distress unrelieved by • No tachypnea for age • Symptoms • Symptoms not noninvasive • Pulse ox >/= 90% RA • manageable with bulb manageable with bulb O2 PRN if SpO2 <88% Reassess • Feeding well • Antipyretics PRN suction only (or nose suction only (or nose • Consider PO Trial (Table 1) frida if available) frida if available) Bulb suction or Reassess Assess qualification for Severe NoseFrida teaching Yes (Table 1) Home O2 Anticipatory guidance (see protocol) DC home Moderate • Adjust O2 flow PRN for