A Study of Ground Water Quality of Anna Nagar West Extension
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thiruvallur District
DISTRICT DISASTER MANAGEMENT PLAN FOR 2017 TIRUVALLUR DISTRICT tmt.E.sundaravalli, I.A.S., DISTRICT COLLECTOR TIRUVALLUR DISTRICT TAMIL NADU 2 COLLECTORATE, TIRUVALLUR 3 tiruvallur district 4 DISTRICT DISASTER MANAGEMENT PLAN TIRUVALLUR DISTRICT - 2017 INDEX Sl. DETAILS No PAGE NO. 1 List of abbreviations present in the plan 5-6 2 Introduction 7-13 3 District Profile 14-21 4 Disaster Management Goals (2017-2030) 22-28 Hazard, Risk and Vulnerability analysis with sample maps & link to 5 29-68 all vulnerable maps 6 Institutional Machanism 69-74 7 Preparedness 75-78 Prevention & Mitigation Plan (2015-2030) 8 (What Major & Minor Disaster will be addressed through mitigation 79-108 measures) Response Plan - Including Incident Response System (Covering 9 109-112 Rescue, Evacuation and Relief) 10 Recovery and Reconstruction Plan 113-124 11 Mainstreaming of Disaster Management in Developmental Plans 125-147 12 Community & other Stakeholder participation 148-156 Linkages / Co-oridnation with other agencies for Disaster 13 157-165 Management 14 Budget and Other Financial allocation - Outlays of major schemes 166-169 15 Monitoring and Evaluation 170-198 Risk Communications Strategies (Telecommunication /VHF/ Media 16 199 / CDRRP etc.,) Important contact Numbers and provision for link to detailed 17 200-267 information 18 Dos and Don’ts during all possible Hazards including Heat Wave 268-278 19 Important G.Os 279-320 20 Linkages with IDRN 321 21 Specific issues on various Vulnerable Groups have been addressed 322-324 22 Mock Drill Schedules 325-336 -
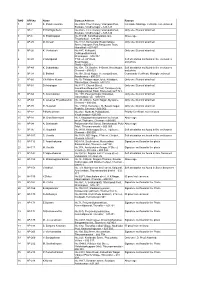
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -

PAN Card Centre Or Offices in Anna Nagar
PAN Card Centre or Offices in Anna Nagar Code of Facilitator Email Communication Contact Address of TIN FC with a PAN Card Office 6070 Karvy Data tinannanaga H Senthil Kumar Pan Card Contact Management [email protected] Address: T-92, Services Ltd. m Ground Floor, Third Avenue Opposite Mosque, Anna Nagar, Chennai, Tamil Nadu- 600040 Tel:44--26220112 3174 Integrated Data annanagaret S Balaji Y Pan Card Facilitator: Management ds@integrat Muralidharan S W-65, A1, TLV Services Private edindia.in Palani Manor, Ground Floor, Ltd. Anna Nagar, Chennai, Tamil Nadu- 600040 Tel:44-- 26211749,984090823 1 14841 Alankit Ltd. rickydz@ho Nikhil Dsouza Pan Card Center: tmail.com Block D-82, Friends Colony, Anna Nagar, East Chennai, Chennai Tamil Nadu- 600102 Tel:44-- 26641719,984112214 1 14429 Alankit Ltd. shanmugara P Shanmugaraj Pan Card Centres: j_p@yahoo. Old Number 22, New com; No.50, Near Anna shanmugara Nagar Railway [email protected] Station, Navallar m Nagar, Thirumangalam Road, Anna Nagar (west), Chennai, Tamil Nadu- 600040 Tel:44-- 43576564,984083274 0 34744 Steel City cscjagan.20 Jaganathan P Pan Card Office: Securities Ltd. [email protected] Common Service om Center, Door Number 5/16, Round Building, Anna Nagar West Extn, Chennai, Tamil Nadu- 600101 Tel:44--7010635299 3805 Integrated Data annanagarw Raja Lakshmi A Pan Card Office Management estetds@int Address: W-596/G1, Services Private egratedindia Ground Floor Absara Ltd. .in Manor, Park Road, Anna Nagar West Extn., Chennai, Tamil Nadu- 600101 Tel:44-- 26152440,735817171 5 8445 Vertex Customer Nnagaraj@t N Pan Card Office Solutions India axmanindia. -

Advance Tour Programme for Division Fever Camps 17.04.2021
Advance Tour Programme for Division Fever Camps 17.04.2021 FIRST CAMP LOCATION SECOND CAMP LOCATION THIRD CAMP LOCATION SNO ZONE DIVISION MO POSTED 8.30 AM TO 11 AM 11.30 AM TO 1.30 PM 4.00 AM TO 7.00 PM 1 4 Magaliamman Kovil street , Ernavoor , Perumal kovil street , Ernavoor , Chennai Dr.Prasath 2 2 Thilagar Nagar , Ennore , Chennai 57 Kamraj Nagar , , Ennore , Chennai 57 Dr.Arun Vivekanandar 3 11 Kailash Sector , Thiruvottiyur , Chennai 19 Janakiyammal Estate , Thiruvottiyur , Dr.Arunya Thiruvottiyur 4 12 & 13 Sathangadu High Road , ( Near St.Paul's School Ayyapillaithottam 2nd Street , Dr.Vijiyalakshmi 5 9 Thiruvottiyur High road , Thiruvottiyur , Pattinathar Kovil street , , Thiruvottiyur , Dr.Vinodh 6 8 Masthan kovil Street , Thiruvottiyur Dr.Saranyavathi 7 19 1st Main road , Mathur, Manali 3rd main road, Mathur, Manali . Dr.Benith Manali 8 18 Thillaipuram, Manali Div.20. New MGR Nagar, Manali Dr. Elumalai 9 22 GANDHI MAIN STREET DR. RAMESH RAJA 10 24 SIVA PRAKASAM NAGAR DR. RAMESH RAJA 11 25 KATTIDA THOLLILALAR NAGAR DR. CHRISTINA 12 26 THIRUMALAI NAGAR SASTHRI NAGAR 1. DR. MADUBALA 13 27 IDAIMA NAGAR DR. PRAVEEN KUMAR 14 Madhavaram 28 ARINGAR ANNA STREET DR. NIRMAL KUMAR 15 29 DEVARAJAN STREET DR. SATHYA DEVI 16 30 KAMARAJ STREET DR. PAUL JAISON 17 31 MUTHUMARIAMMAN KOIL STREET DR. ACHINA TITUS 18 32 SARATHY NAGAR 1ST STREET DR. VIDHYA 19 33 KAMARAJ SALAI DR. MYTHILI 20 34 R V NAGAR SOLAIAMMAN KOIL STREET DR. STEPHEN 21 35 T H ROAD CHINNADIMADAM MUTHAMIL NAGAR 7TH BLOCK ICDS Dr.Mathina, 22 36 MKB NAGAR BUS DEPO INDUSTRIAL ESTATE Dr.Thahaseen 23 37 MULLAI COMPLEX VALLUVAR STREET Dr.Joseph 24 38 NETHAJI NAGAR MAIN ROAD NETHAJI NAGAR 5TH ST Dr.Karan 25 39 A E SCHEME ROAD NAGOORAR THOTTAM Dr.Thirumalai raj 26 40 CORPORATION COLONY T SUNAMI QUATETRS Dr.kalaiyarasi 27 Tondiarpet 41 ANNA NAGAR DEVIKARUMARIAMMAN NAGAR DR.Zoharath 28 42 T H ROAD MAIN ST THILAGAR NAGAR COMMUNITY HALL DR. -

IEPF-2 Disclosures FY-17-18
TCP Ltd Details of Unclaimed deposits as at the 46th AGM date 26-10-18 For the Financial year ended 31-3-18 (1st Year for IEPF Rule 5 (8) compliance) Unclaimed position as at the 46th AGM held on 26-10-18 - 1st year intimation (the unclaimed status details of the following depositors shall be furnished as at the closure of each of the succeeding 7 AGM's up to the AGM held for the Financial year ended 31-3-25) For the purpose of filing Form IEPF-2 And thereafter it should be remitted to the IEPF within 30 days from the due date Fixed Deposit Serial No. Name Address Nature of Amount Amount Rs. Due date for transfer FDR No. to the IEPF 1 Rajalakshmi Natarajan 28/2, Lakshmi Colony, T. Nagar, Matured deposit 10,000 12/6/2017 16376 Chennai 600017 2 P. Eswaramurthy Old No.36, New No.54, Krishnappa Matured deposit 10,000 5/1/2018 16517 Agraharam Street, Chennai 600079 3 N. Krishna Kumar New No.10, Old No.41, IInd Street, Matured deposit 20,000 8/17/2018 16908 Parthasarathy Nagar, Adambakkam, Chennai 600088 4 L. Janaki 23/48, Rani Anna Nagar, Matured deposit 20,000 9/3/2018 16989 K K Nagar, Chennai 600078 5 Nalini R Pai No.13, A G Colony, Matured deposit 16,000 3/14/2019 17428 Alwar Thirunagar, Chennai 600087 6 P. Ravisekar 24/19, Second Main Road, J B Estate Matured deposit 10,000 11/23/2019 18311 Avadi, Chennai 600054 7 Vasantha Ravindran 1 Sri Devi Apartments, Matured deposit 25,000 5/23/2020 18638 5/2, 4th Trust Cross Street, Mandaveli, Chennai 600028 8 Hemlatha D Tarvady Shri Shraddha', 5/2A, Dr. -

Chennai North Commissionerate Jurisdiction
Chennai North Commissionerate Jurisdiction The jurisdiction of Chennai North Commissionerate will cover the areas covering Chennai Corporation Zone Nos. I to IX (From Ward Nos. I to 126 in existence as on OL-O4-2OL7) in the State of Tamil Nadu. The Continental shelf and exclusive economic zone contiguous to the eastern coast of India. No.26l1, Mahathma Gandhi Road, Nungambakkam, Location Chennai 600 O34 Divisions under the jurisdiction of Chennai North Commissionerate. Sl.No. Divisions 1, Thiruvottiyur Division 2. Madhavaram Division 3. Royapuram Division 4. Parrys Division 5. Egmore Division 6. Thiru Vi Ka Nagar Division 7. Ambattur Division 8. Anna Nagar Division 9. Pursawalkam Division 10. Nungambakkam Division 11. Triplicane Division L2. Mylapore Division Page 5 of 83 l.Thiruvottirnrr Division of Chennai North Commissionerate Location Ananda Complex, 459, Anna Salai, Te5rnampet, Chennai - 600 018 Jurisdiction Areas covering Ward Nos. 1 to L4 of Zone I & Ward Nos. 15 to 2Iof Zone II of Chennai Corporation, Taxpayers names starting with letters A, B, Y and Z of the Continental shelf and exclusive economic zone contiguous to the eastern course of India. The Division has five Ranges with jurisdiction as follows: Name of the Range Location Jurisdiction Areas covering ward Nos. L to 4 of Zone I, Taxpayers names starting with letters A of the Range I Continental shelf and exclusive econornic zone contiguous to the eastern course of India. Areas covering ward Nos. 5 to 9 of Zone I, Range II Taxpayers names starting with letters B of the Ananda Continental shelf and exclusive econornic zone Complex, contiguous to the eastern course of India. -
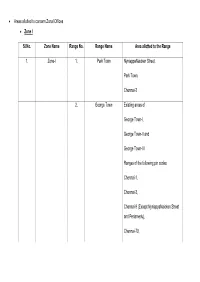
• Areas Allotted to Concern Zonal Offices • Zone I Sl.No. Zone Name Range No. Range Name Area Allotted to the Range 1. Zone
• Areas allotted to concern Zonal Offices • Zone I Sl.No. Zone Name Range No. Range Name Area allotted to the Range 1. Zone-I 1. Park Town NyniappaNaicken Street, Park Town, Chennai-3 2. George Town Existing areas of George Town-I, George Town-II and George Town-III Ranges of the following pin codes: Chennai-1, Chennai-3, Chennai-9 (Except NyniappaNaicken Street and Periamedu), Chennai-79, Chennai-108. 3. Tondiarpet-I Existing Tondiarpet-I areas of Chennai Corporation. 4. Tondiarpet-II Existing Tondiarpet-II areas of Chennai Corporation. 5. Egmore Chennai Corporation postal pin code: Chennai-8, Chennai-34 and Periamedu, Chennai-3. 6. Veppery Chennai Corporation postal pin code: Chennai-7, Chennai-112. 7. Perambur Chennai Corporation postal pin code: Chennai-11, Chennai-12. 8. Vysarpadi Chennai Corporation postal pin code: Chennai-118, Chennai-39 and Chennai-51. Zone-II Sl.No. Zone Name Range No. Range Name Area allotted to the Range 2. Zone-II 9. Arumbakkam Chennai Corporation postal pin code: Arumbakkam, Chennai-106, Ammjikarai, Chennai-29, Koyembedu, Chennai-107. 10. Anna Nagar Chennai Corporation postal pin code: Anna Nagar, Chennai-4, Shenoy Nagar, Chennai-30, Anna Nagar West Extn., Chennai-101. Of Pre-extended Chennai Corporation (of Chennai Revenue District). 11. Ayanavaram Chennai Corporation postal pin code: Ayanavaram, Chennai-23, Periyar Nagar, Chennai-82. 12. Villivakkam Chennai Corporation postal pin code: Villivakkam, Chennai-49, Kolathur, Chennai-99, Anna Nagar East, Chennai-102. 13. Kilpauk Chennai Corporation postal pin code: Kilpauk, Chennai-10, Flowers Road, Purasawakkam.. 14. Kodambakkam Chennai Corporation postal pin code: Kodambakkam, Chennai-24, Choolaimedu, Chennai-94. -
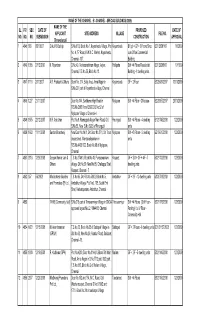
Sl. No. Pp No. Sbc No. Date of Submission Name Of
NAME OF THE CHANNEL : B - CHANNEL - SPECIAL BUILDINGS (2009) NAME OF THE SL. P.P. SBC DATE OF PROPOSED DATE OF APPLICANT SITE ADDRESS VILLAGE FILE NO. NO. NO. NO. SUBMISSION CONSTRUCTION APPROVAL (Thiruvalargal) 1 4845 889 8/31/2007 Gokul R. Bathija S.No.61/2, Block No.1, Koyambedu Village, Plot Koyambedu BF(pt) + GF + 3 Floors Shop BC1/20301/07 1/6/2009 No. 4, "E" Road, K.W.M.C. Market, Koyambedu, cum Office Commercial Chennai - 107. Building 2 4846 1365 27/12/2007 K. Rajendran D.No.49, Venkatarathinam Nagar, Adyar, Pallipattu Stilt + 4 Floors Residential BC1/29398/07 1/7/1909 Chennai, T.S.No.56, Block No.12. Building - 8 dwelling units 3 4847 1118 2/11/2007 A.R. Prakash & Others Block No .314, Belly Area, Anna Nagar in Koyambedu GF + 2 Floor B3/25684/2007 13/01/2009 S.No.207 part of Koyambedu village, Chennai 4 4848 1227 21/11/2007 Door No.144, Santhome High Road in Mylapore Stilt + 4 Floor - Office use B2/26857/2007 20/01/2009 R.S.No.2593/1 and 2593/2, B.No.52 of Mylapore Village of Chennai-4 5 4849 1975 22/12/2007 K.R. Aniruthan Plot No.9, Ramappah Nagar Main Road, Old Perungudi Stilt + 4 Floors - 4 dwelling B1/21745/2008 1/22/2009 S.No.56, New S.No.56/26 of Perungudi units 6 4850 1692 11/11/2008 Sundar Bharatwaj New Door No.14/1, Old Door No.37/1, 5th Trust Mylapore Stilt + 3 Floors - 5 dwelling B2/19610/2008 1/22/2009 cross street, Mandavellipakkam in units R.S.No.4436/172, Block No.98 of Mylapore, Chennai 7 4851 2018 12/26/2008 Deepak Kumar Jain & T.S.No.2749/1, Block No.40, Purasawakkam Kosapet GF + Stilt + 3 F + 4 F - 7 B3/21733/2008 1/22/2009 Others village, Old No.30, New No.85, Chellappa Stret, dwelling units Kosapet, Chennai - 7. -

Tamil Nadu Government Gazette
© GOVERNMENT OF TAMIL NADU [Regd. No. TN/CCN/467/2009-11. 2009 [Price : Rs. 170. 40 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 28B] CHENNAI, WEDNESDAY, JULY 22, 2009 Aadi 6, Thiruvalluvar Aandu–2040 Part VI–Section 1 (Supplement) NOTIFICATIONS BY HEADS OF DEPARTMENTS, ETC. TAMIL NADU DENTAL COUNCIL NOTICE OF ELECTION TO THE DENTAL COUNCIL OF INDIA, NEW DELHI AND TO THE TAMIL NADU DENTAL COUNCIL, CHENNAI (Ref. No. TNDC/GCP/PER/09-2.) No. VI(1)/218/2009. Notice under Dental Council of India Election Regulations, 1952—Section 3(3) AND Notice under Tamil Nadu Dental Council Rules—G.O. No. 2638/Health and Family Welfare Department/Government of Tamil Nadu, dated 24-07-1950 — Election Rules — Section 2(3) Election of one member to the Dental Council of India under Section 3(a) AND Election of four members to the Tamil Nadu Dental Council under Section 21(a) AND Election of four members to the Tamil Nadu Dental Council under Section 21(b) of The Dentists Act, 1948. The Preliminary Electoral Roll as on 30th June 2009, showing the particulars of names of the registered Dentists to vote in the elections mentioned above, is published in the Tamil Nadu Government Gazette, Issue No. 28B, dated 22nd July 2009. Claims and Objections relating to the entries or omissions in the Preliminary Electoral Roll, with proof, shall be sent by registered post only to the undersigned on or before 31st August 2009 at 5 p.m. Claims and Objections received after the said date will not be accepted. -

Abuthahir". Our Office Address : No.1325,18Th Main Road, Anna
LOT business (Bulk Sales & Purchase of all items) Thanks for your interest and support. We would like to give some brief introduction about my Company and purpose of getting Investment. The company "AL FATTAH ENTERPRISES" is a Proprietorship firm and it is registered under my name "Syed Abuthahir". Our Office Address : No.1325,18th Main Road, Anna Nagar West, Chennai - 600040 and Godown is located at No.8, Kasiammal Nagar, Ponniammanmedu, Madhavaram, Chennai - 600 0110. The business we are handling is LOT business (Bulk Sales & Purchase of all items) for more than 5 years and we have registered our company 2 years back. Actually Iam running the business along with my partners, since they are doing this business for long time and we have made internal partnership agreement. The purpose of getting new Investments is to develop our business and to take it to next level. At present we are doing 20% of our business transactions through bank and balance 80% of transactions through cash. To know better, please find below businesses we were handled in past 2 years • We deal with fresh and outdated model of Garments and Shoes especially Reebok, Nike, Lancer Shoes, Aravind Mill Garments, Toys, etc. • We bought Usha company manufactured items (Sewing Machines and many more) which was affected in the flood and sold out. • We bought Butterfly company manufactured items (Table & Ceiling Fans) which was affected in the flood and sold out. • We bought Lifestyle company manufactured items (Garments) 1 to 2 year old stock and sold out. • We bought Hitachi company manufactured items (Air-Conditions) which was affected in the flood and sold out. -
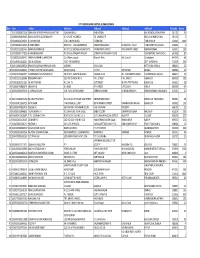
IEPF SHARE TRANSFERED NOV 2017.Xlsx
CITY UNION BANK LIMITED, KUMBAKONAM Slno Folio Holder Address1 Address2 Address3 Address4 Pin Code Shares 1 1201320000673324 SHARADA VEERAPPA FARALASHATTAR JIGAJINNI GALLI BAILHONGAL BAILHONGAL KARNATAKA 591102 10 2 1203840000658455 MANJUNATH D GUDENNAVAR AT & POST. UGARGOL TQ. SAUNDATTI BELGAUM KARNATAKA 591126 5 3 IN30039411792259 K CHINNAIYAN 3/36 B MAIN ROAD 53 SIMIZHI THIRUVARUR 612603 1000 4 1201090003109515 K RAJENDRAN . NEW NO I- 143,AGRAHARAM ARASAVANAGKADU KUDAVASAL TALUK TIRUVARUR TAMILNADU 612603 4 5 IN30131321181443 RAMACHANDRAN D NO 57 C/3 SEETHALAKSHMI APTS PACHAYAPPA STREET PACHAYAPPA STREET KUMBAKONAM 612001 250 6 1201090001777855 R ANANDAKUMAR . NO 9 KARUPPASAMY NAGAR SOWRIPALAYAM MAIN ROAD COIMBATORE TAMILNADU 641028 87 7 IN30223611395432 AMBALAVANAN KUMARESAN 11-A Remy Layout Bharathi Park, 3rd Cross St Coimbatore 641043 60 8 IN30069310102281 ARUN GOPALKA POST- SITARAMPUR DIST- BURDWAN 713359 550 9 1201130000228524 PRAVEEN GOPALAKRISHNAN NAIR AMBADY, CHUNGAM, KOTTAYAM KERALA 686001 25 10 IN30169611286403 THOMAS KURIAN KADAPLACKAL KADAPLACKAL TEEKOY KOTTAYAM KERALA 686580 30 11 IN30148510664297 CHAKRAPANI GOVINDAKURUP MP V/217, SARATH BHAVAN VAZHAKULAM PO - MADAKKATHANAM THODUPUZHA, KERALA 686671 45 12 IN30131321262686 RENGANATHAN P OLD NO 29 NEW NO 5 P A L STREET P A L STREET KARAIKUDI 630001 200 13 IN30108022721003 AL MEYYAPPAN NO 104 75 1 ST STREET MUTHUPPATTINAM, KARAIKUDI 630001 38 14 IN30163740883679 ABINAYA G NO 95/B 4 TH CROSS S P COLONY KARUR 639004 64 15 1203840000303191 K. ARIVAZHAGAN . 526, PUDU AGRAHARAM -

List of the Candidates Short Listed for the Post of Office Assistant to Attend the Interview at Principal District Court, Ariyalur
LIST OF THE CANDIDATES SHORT LISTED FOR THE POST OF OFFICE ASSISTANT TO ATTEND THE INTERVIEW AT PRINCIPAL DISTRICT COURT, ARIYALUR 8.MBC&DC – General – Non Priority 63, 67, 83 & 87 Sl. Application Name and Address Date & Time No. No. Sivakumar.R, s/o Ramalingam, keelanatham, 10.04.2018 1 Employment Ambavur post, 9.00 A.M Udayarpalayam Taluk, Ariyalur. Selvarajadurai.P, s/o Palanisamy, South street, 10.04.2018 2 Employment Kodali post, 9.00 A.M Udayarpalayam Taluk, Ariyalur. Natarajan.P, s/o Perumal, 10.04.2018 3 Employment Irumbulikurichy post, 9.00 A.M Sendurai Taluk, Ariyalur. Lenin.R, s/o Rajayokiam, Kusalaganpatty, 10.04.2018 4 Employment Ponparappi, 9.00 A.M Sendurai Taluk, Ariyalur. Raja.P, s/o Panjanathan, 10.04.2018 5 Employment No.10B, Keela street, 9.00 A.M Ariyalur. Jayaprakash.M, s/o Mathiyalagan, 1/79 North street, 10.04.2018 6 Employment Thenkachiperumal, 9.00 A.M Natham post, Udayarpalayam Taluk, Ariyalur. Jayasankar.D, M80, New Housing Unit, 10.04.2018 7 Employment Lakshmi mahal backside, 9.00 A.M Kurumban savadi, Ariyalur. Nallamuthu.K, s/o Kathaperumal, 10.04.2018 8 Employment Kumiliyam post, 9.00 A.M Ariyalur. Murugan.D, s/o Dharmalingam, 10.04.2018 9 Employment 1A1, Purushothama vathiyar street, 9.00 A.M Ariyalur. Prabu.S, D/o Shanmugasundaram, 10.04.2018 10 Employment Ponparappi post, 9.00 A.M Sendurai Taluk, Ariyalur. Velmurugan.A, 3/20 west street, 10.04.2018 11 Employment Kudukur post, 9.00 A.M Udayarpalayam Taluk, Ariyalur. Velmurugan.K, s/o kaliyaperumal, South street, 10.04.2018 12 Employment Anaikudam post, 9.00 A.M Udayarpalayam Taluk, Ariyalur.